Validation of a Multiplex Molecular Macroarray for the Determination of Allergen-Specific IgE Sensitizations in Dogs
- PMID: 39453074
- PMCID: PMC11512340
- DOI: 10.3390/vetsci11100482
Validation of a Multiplex Molecular Macroarray for the Determination of Allergen-Specific IgE Sensitizations in Dogs
Abstract
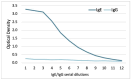
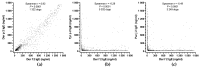
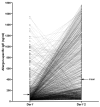
Detecting IgE sensitizations in the serum of allergic dogs is commonly performed using allergen extracts, but these are difficult to standardize. This article details the development and validation of the Pet Allergy Xplorer (PAX; Nextmune, Stockholm, Sweden), the first multiplex macroarray for the detection of IgE sensitization in dogs using allergen extracts and molecular components; the PAX is derived from the Allergy Xplorer (ALEX2; MacroArray Diagnostics, Vienna, Austria). The selection of allergens, cartridge processing, strategy for identifying and blocking IgE directed against cross-reactive carbohydrate determinants (CCDs), and the method used for determining the positivity threshold are described. The validation of the PAX included evaluations of the specificity of its anti-IgE monoclonal antibody, specificity of IgE binding to target allergens, assay precision, and internal consistency. Additionally, the influence of possible confounding factors, such as sample type, the influence of hemolysis, lipemia, bilirubinemia, and elevated CCD-IgE, was tested. Finally, the sensitization rates of 23,858 European dogs to 145 environmental and Hymenoptera venom allergens were summarized. The PAX is accurate and reproducible and has a unique CCD-detection and blocking strategy; its molecular allergens offer a unique window on allergen cross-reactivity.
Keywords: IgE; allergen; allergy; atopic dermatitis; dog; molecular allergology; serodiagnosis; serology.
Conflict of interest statement
T.O. and A.M.F. work for Nextmune, which commercializes the PAX, while M.A., N.P.I., G.M., and C.H. are employed by MacroArray Diagnostics, which manufactures both ALEX2 and the PAX.
Figures


References
LinkOut - more resources
Full Text Sources

