A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning
- PMID: 39453188
- PMCID: PMC11510859
- DOI: 10.3390/toxins16100412
A Monoclonal Antibody with a High Affinity for Ricin Isoforms D and E Provides Strong Protection against Ricin Poisoning
Abstract
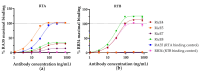
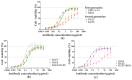
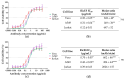
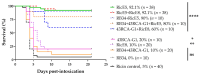
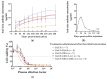
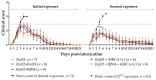
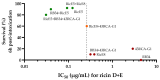
Ricin is a highly potent toxin that has been used in various attempts at bioterrorism worldwide. Although a vaccine for preventing ricin poisoning (RiVax™) is in clinical development, there are currently no commercially available prophylaxis or treatments for ricin intoxication. Numerous studies have highlighted the potential of passive immunotherapy using anti-ricin monoclonal antibodies (mAbs) and have shown promising results in preclinical models. In this article, we describe the neutralizing and protective efficacy of a new generation of high-affinity anti-ricin mAbs, which bind and neutralize very efficiently both ricin isoforms D and E in vitro through cytotoxicity cell assays. In vivo, protection assay revealed that one of these mAbs (RicE5) conferred over 90% survival in a murine model challenged intranasally with a 5 LD50 of ricin and treated by intravenous administration of the mAbs 6 h post-intoxication. Notably, a 35% survival rate was observed even when treatment was administered 24 h post-exposure. Moreover, all surviving mice exhibited long-term immunity to high ricin doses. These findings offer promising results for the clinical development of a therapeutic candidate against ricin intoxication and may also pave the way for novel vaccination strategies against ricin or other toxins.
Keywords: long-term immunity; monoclonal antibodies; neutralizing antibodies; passive immunotherapy; ricin; ricin isoforms.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Schieltz D.M., McWilliams L.G., Kuklenyik Z., Prezioso S.M., Carter A.J., Williamson Y.M., McGrath S.C., Morse S.A., Barr J.R. Quantification of Ricin, RCA and Comparison of Enzymatic Activity in 18 Ricinus communis Cultivars by Isotope Dilution Mass Spectrometry. Toxicon. 2015;95:72–83. doi: 10.1016/j.toxicon.2015.01.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

