From Venom to Vein: Factor VII Activation as a Major Pathophysiological Target for Procoagulant Australian Elapid Snake Venoms
- PMID: 39453206
- PMCID: PMC11510989
- DOI: 10.3390/toxins16100430
From Venom to Vein: Factor VII Activation as a Major Pathophysiological Target for Procoagulant Australian Elapid Snake Venoms
Abstract
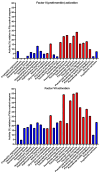
Australian elapid snake venoms are uniquely procoagulant, utilizing blood clotting enzyme Factor Xa (FXa) as a toxin, which evolved as a basal trait in this clade. The subsequent recruitment of Factor Va (FVa) as a toxin occurred in the last common ancestor of taipans (Oxyuranus species) and brown snakes (Pseudonaja species). Factor II (prothrombin) activation has been stated as the primary mechanism for the lethal coagulopathy, but this hypothesis has never been tested. The additional activation of Factor VII (FVII) by Oxyuranus/Pseudonaja venoms has historically been considered as a minor, unimportant novelty. This study aimed to investigate the significance of toxic FVII activation relative to prothrombin activation by testing a wide taxonomical range of Australian elapid species with procoagulant venoms. The activation of FVII or prothrombin, with and without the Factor Va as a cofactor, was assessed, along with the structural changes involved in these processes. All procoagulant species could activate FVII, establishing this as a basal trait. In contrast, only some lineages could activate prothrombin, indicating that this is a derived trait. For species able to activate both zymogens, Factor VII was consistently more strongly activated than prothrombin. FVa was revealed as an essential cofactor for FVII activation, a mechanism previously undocumented. Species lacking FVa in their venom utilized endogenous plasma FVa to exert this activity. The ability of the human FXa:FVa complex to activate FVII was also revealed as a new feedback loop in the endogenous clotting cascade. Toxin sequence analyses identified structural changes essential for the derived trait of prothrombin activation. This study presents a paradigm shift in understanding how elapid venoms activate coagulation factors, highlighting the critical role of FVII activation in the pathophysiological effects upon the coagulation cascade produced by Australian elapid snake venoms. It also documented the novel use of Factor Va as a cofactor for FVII activation for both venom and endogenous forms of FXa. These findings are crucial for developing better antivenoms and treatments for snakebite victims and have broader implications for drug design and the treatment of coagulation disorders. The research also advances the evolutionary biology knowledge of snake venoms.
Keywords: Factor VII; Factor Va; adaptation; coagulation; evolutionary biology; molecular evolution; prothrombin; venom; zymogen.
Conflict of interest statement
Nathan Dunstan was employed by the Venom Supplies Pty Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms.Toxins (Basel). 2025 Aug 18;17(8):417. doi: 10.3390/toxins17080417. Toxins (Basel). 2025. PMID: 40864093 Free PMC article.
-
Clinical implications of convergent procoagulant toxicity and differential antivenom efficacy in Australian elapid snake venoms.Toxicol Lett. 2019 Nov;316:171-182. doi: 10.1016/j.toxlet.2019.08.014. Epub 2019 Aug 20. Toxicol Lett. 2019. PMID: 31442586
-
Keel venom: Rhabdophis subminiatus (Red-Necked Keelback) venom pathophysiologically affects diverse blood clotting pathways.Toxicon. 2022 Oct 30;218:19-24. doi: 10.1016/j.toxicon.2022.08.017. Epub 2022 Aug 31. Toxicon. 2022. PMID: 36057394
-
Procoagulant adaptation of a blood coagulation prothrombinase-like enzyme complex in australian elapid venom.Toxins (Basel). 2010 Jun;2(6):1554-67. doi: 10.3390/toxins2061554. Epub 2010 Jun 18. Toxins (Basel). 2010. PMID: 21127733 Free PMC article. Review.
-
Factor Va-factor Xa interactions: molecular sites involved in enzyme:cofactor assembly.Scand J Clin Lab Invest Suppl. 2002;237:5-11. doi: 10.1080/003655102762377439. Scand J Clin Lab Invest Suppl. 2002. PMID: 12570161 Review.
Cited by
-
Snake Venom Makeover: Age-Dependent Variations in Procoagulant Biochemistry of Egyptian Saw-Scaled Viper (Echis pyramidum pyramidum) Venom.Toxins (Basel). 2025 Mar 19;17(3):149. doi: 10.3390/toxins17030149. Toxins (Basel). 2025. PMID: 40137922 Free PMC article.
-
Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species).Toxins (Basel). 2025 Aug 8;17(8):398. doi: 10.3390/toxins17080398. Toxins (Basel). 2025. PMID: 40864074 Free PMC article.
-
X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms.Toxins (Basel). 2025 Aug 18;17(8):417. doi: 10.3390/toxins17080417. Toxins (Basel). 2025. PMID: 40864093 Free PMC article.
-
Age Is Just a Number: Ontogenetic Conservation in Activation of Blood Clotting Factors VII, X, and XII by Caucasus Blunt-Nosed Viper (Macrovipera lebetina obtusa) Venoms.Toxins (Basel). 2024 Dec 2;16(12):520. doi: 10.3390/toxins16120520. Toxins (Basel). 2024. PMID: 39728778 Free PMC article.
References
-
- Casewell N.R., Sunagar K., Takacs Z., Calvete J.J., Jackson T.N.W., Fry B.G. Snake venom metalloprotease enzymes. In: Fry B.G., editor. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford University Press; New York, NY, USA: 2015. pp. 347–363.
-
- Debono J., Dobson J., Casewell N.R., Romilio A., Li B., Kurniawan N., Mardon K., Weisbecker V., Nouwens A., Kwok H.F., et al. Coagulating colubrids: Evolutionary, pathophysiological and biodiscovery implications of venom variations between Boomslang (Dispholidus typus) and Twig Snake (Thelotornis mossambicanus) Toxins. 2017;9:171. doi: 10.3390/toxins9050171. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

