Neuronal cathepsin S increases neuroinflammation and causes cognitive decline via CX3CL1-CX3CR1 axis and JAK2-STAT3 pathway in aging and Alzheimer's disease
- PMID: 39453382
- PMCID: PMC11822647
- DOI: 10.1111/acel.14393
Neuronal cathepsin S increases neuroinflammation and causes cognitive decline via CX3CL1-CX3CR1 axis and JAK2-STAT3 pathway in aging and Alzheimer's disease
Abstract
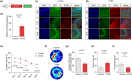
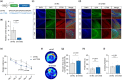
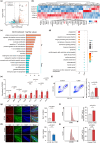
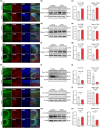
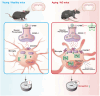
Aging is an intricate process involving interactions among multiple factors, which is one of the main risks for chronic diseases, including Alzheimer's disease (AD). As a member of cysteine protease, cathepsin S (CTSS) has been implicated in inflammation across various diseases. Here, we investigated the role of neuronal CTSS in aging and AD started by examining CTSS expression in hippocampus neurons of aging mice and identified a significant increase, which was negatively correlated with recognition abilities. Concurrently, we observed an elevation of CTSS concentration in the serum of elderly people. Transcriptome and fluorescence-activated cell sorting (FACS) results revealed that CTSS overexpression in neurons aggravated brain inflammatory milieu with microglia activation to M1 pro-inflammatory phenotype, activation of chemokine C-X3-C-motif ligand 1 (CX3CL1)-chemokine C-X3-C-motif receptor 1 (CX3CR1) axis and janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) pathway. As CX3CL1 is secreted by neurons and acts on the CX3CR1 in microglia, our results revealed for the first time the role of neuron CTSS in neuron-microglia "crosstalk." Besides, we observed elevated CTSS expression in multiple brain regions of AD patients, including the hippocampus. Utilizing CTSS selective inhibitor, LY3000328, rescued AD-related pathological features in APP/PS1 mice. We further noticed that neuronal CTSS overexpression increased cathepsin B (CTSB) activity, but decreased cathepsin L (CTSL) activity in microglia. Overall, we provide evidence that CTSS can be used as an aging biomarker and plays regulatory roles through modulating neuroinflammation and recognition in aging and AD process.
Keywords: Alzheimer's disease; aging; cathepsin S; neurodegenerative disease; neuroinflammation; recognition.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
-
- Agrawal, S. , Baulch, J. E. , Madan, S. , Salah, S. , Cheeks, S. N. , Krattli, R. P. , Subramanian, V. S. , Acharya, M. M. , & Agrawal, A. (2022). Impact of IL‐21‐associated peripheral and brain crosstalk on the Alzheimer's disease neuropathology. Cellular and Molecular Life Sciences, 79, 331. - PMC - PubMed
-
- Akiyama, H. , Arai, T. , Kondo, H. , Tanno, E. , Haga, C. , & Ikeda, K. (2000). Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Disease and Associated Disorders, 14(1), 47–53. - PubMed
-
- Akiyama, H. , Barger, S. , Barnum, S. , Bradt, B. , Bauer, J. , Cole, G. M. , Cooper, N. R. , Eikelenboom, P. , Emmerling, M. , Fiebich, B. L. , Finch, C. E. , Frautschy, S. , Griffin, W. S. , Hampel, H. , Hull, M. , Landreth, G. , Lue, L. , Mrak, R. , Mackenzie, I. R. , … Wyss‐Coray, T. (2000). Inflammation and Alzheimer's disease. Neurobiology of Aging, 21, 383–421. - PMC - PubMed
-
- Aunan, J. R. , Watson, M. M. , Hagland, H. R. , & Søreide, K. (2016). Molecular and biological hallmarks of ageing. The British Journal of Surgery, 103, e29–e46. - PubMed
-
- Biswas, K. (2023). Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. Journal of Neuroimmunology, 383, 578180. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

