Midface Projection Using Biostimulatory Poly- l -Lactic Acid Injectable Implant: A Subgroup Analysis of the Cheek Wrinkle Trial
- PMID: 39453400
- PMCID: PMC11593986
- DOI: 10.1097/DSS.0000000000004434
Midface Projection Using Biostimulatory Poly- l -Lactic Acid Injectable Implant: A Subgroup Analysis of the Cheek Wrinkle Trial
Abstract
Background: Correction of cheek wrinkles using poly- l -lactic acid (PLLA-SCA) was demonstrated in a 12-month study.
Objective: This analysis assessed change from baseline in lifting effect of PLLA-SCA using a 3D camera to provide additional quantified data.
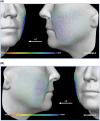
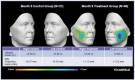
Methods: Subjects received PLLA-SCA (reconstituted in 8 mL of sterile water + 1 mL of 2% lidocaine) in both cheeks or no treatment (control). Assessments included the Galderma Cheek Wrinkle Scale (GCWS), aesthetic improvement, and 3D photography. In subjects with severe GCWS at baseline, Canfield software analyzed 3D images for change from baseline in midface volume projection and volume change of both cheeks at Month 9.
Results: The primary study showed a statistically significant higher at-rest GCWS responder rate at Month 12 for PLLA-SCA, 71.6%, versus control, 26.1% ( p < .0001). In this Month 9 analysis, mean midface volume projection demonstrated a positive volume shift for PLLA-SCA (left: 0.45 mm; right: 0.34 mm; n = 46) versus control (left: 0.25 mm; right: -0.01 mm; n = 21). Midface volume and max projection changes for PLLA-SCA were +4.88 mL/+1.62 mm (left), +2.84 mL/+1.12 mm (right) versus +0.26 mL/+0.34 mm (left), +0.68 mL/+0.37 mm (right) for control.
Conclusion: Poly- l -lactic acid-treatment of subjects with severe GCWS had a clinically meaningful lifting effect in both cheeks (positive volume shift, positive volume change, and max projection) in the midface.
Clinical trial registry number: NCT04124692.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society for Dermatologic Surgery, Inc.
Conflict of interest statement
The authors have indicated no significant interest with commercial supporters.
Figures


References
-
- Sculptra®. US instructions for use. Available from: https://www.sculptrausa.com/docs/Sculptra-e-IFU. Accessed on 1 May 2024.
-
- Alessio R, Rzany B, Eve L, Grangier Y, et al. European expert recommendations on the use of injectable poly-L-lactic acid for facial rejuvenation. J Drugs Dermatol 2014;13:1057–66. - PubMed
-
- Lam SM, Azizzadeh B, Graivier M. Injectable poly-L-lactic acid (Sculptra): technical considerations in soft-tissue contouring. Plast Reconstr Surg 2006;118(3 suppl):55S–63S. - PubMed
-
- Haddad A, Menezes A, Guarnieri C, Coimbra D, et al. Recommendations on the use of injectable poly-L-lactic acid for skin laxity in off-face areas. J Drugs Dermatol 2019;18:929–35. - PubMed
-
- Fabi S, Hamilton T, LaTowsky B, Kazin R, et al. Effectiveness and safety of Sculptra poly-L-lactic acid injectable implant in the correction of cheek wrinkles. J Drugs Dermatol 2024;23:1297–305. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

