Differential effects of myosin activators on myocardial contractile function in nonfailing and failing human hearts
- PMID: 39453428
- PMCID: PMC12239099
- DOI: 10.1152/ajpheart.00252.2024
Differential effects of myosin activators on myocardial contractile function in nonfailing and failing human hearts
Abstract
The second-generation myosin activator danicamtiv (DN) has shown improved function compared with the first-generation myosin activator omecamtiv mecarbil (OM) in nonfailing myocardium by enhancing cardiac force generation but attenuating slowed relaxation. However, whether the functional improvement with DN compared with OM persists in remodeled failing myocardium remains unknown. Therefore, this study aimed to investigate the differential contractile responses to myosin activators in nonfailing and failing myocardium. Mechanical measurements were performed in detergent-skinned myocardium isolated from donor and failing human hearts. Steady-state force, stretch activation responses and loaded shortening velocity were analyzed at submaximal [Ca2+] in the absence or presence of 0.5 µmol/L OM or 2 µmol/L DN. The effects of DN and OM on Ca2+ sensitivity of force generation were determined by incubating myocardial preparations at various [Ca2+]. The inherent impairment in force generation and cross-bridge behavior sensitized the failing myocardium to the effects of myosin activators. Specifically, increased Ca2+ sensitivity of force generation, slowed rates of cross-bridge recruitment and detachment following acute stretch, slowed loaded shortening velocity, and diminished power output were more prominent following treatment with OM or DN in failing myocardium compared with donor myocardium. Although these effects were less pronounced with DN compared with OM in failing myocardium, DN impaired contractile properties in failing myocardium that were not affected in donor myocardium. Our results indicate that similar to first-generation myosin activators, the DN-induced slowing of cross-bridge kinetics may result in a prolongation of systolic ejection and delayed diastolic relaxation in the heart failure setting.NEW & NOTEWORTHY This is the first study to provide a detailed mechanistic comparison of omecamtiv mecarbil (OM) and danicamtiv (DN) in failing and nonfailing human myocardium. These findings have clinical implications and the potential to inform the clinical utility of myosin activators in the heart failure setting.
Keywords: contractile function; danicamtiv; heart failure; myocardium; omecamtiv mecarbil.
Copyright © 2025 The Authors.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




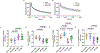




Comment in
-
What frozen human hearts can tell us about treating heart failure.Am J Physiol Heart Circ Physiol. 2025 Jan 1;328(1):H1-H2. doi: 10.1152/ajpheart.00758.2024. Epub 2024 Nov 19. Am J Physiol Heart Circ Physiol. 2025. PMID: 39560014 Free PMC article.
References
-
- Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo-Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, … Kurtz CE Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. New England Journal of Medicine, 384(2), 105–116. 10.1056/NEJMoa2025797 - DOI - PubMed
-
- Fülöp GÁ, Oláh A, Csipo T, Kovács Á, Pórszász R, Veress R, Horváth B, Nagy L, Bódi B, Fagyas M, Helgadottir SL, Bánhegyi V, Juhász B, Bombicz M, Priksz D, Nanasi P, Merkely B, Édes I, Csanádi Z, Papp Z, Radovits T, Tóth A. Omecamtiv mecarbil evokes diastolic dysfunction and leads to periodic electromechanical alternans. Basic Res Cardiol 116: 24, 2021. doi: 10.1007/s00395-021-00866-8. - DOI - PMC - PubMed
-
- Voors AA, Tamby J, Cleland JG, Koren M, Forgosh LB, Gupta D, Lund LH, Camacho A, Karra R, Swart HP, Pellicori P, Wagner F, Hershberger RE, Prasad N, Anderson R, Anto A, Bell K, Edelberg JM, Fang L, Henze M, Kelly C, Kurio G, Li W, Wells K, Yang C, Teichman SL, Rio CL, Solomon SD. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail 22: 1649–1658, 2020. doi: 10.1002/ejhf.1933. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

