Azacitidine in combination with shortened venetoclax treatment cycles in patients with acute myeloid leukemia
- PMID: 39453477
- PMCID: PMC11868173
- DOI: 10.1007/s00277-024-06048-5
Azacitidine in combination with shortened venetoclax treatment cycles in patients with acute myeloid leukemia
Erratum in
-
Correction to: Azacitidine in combination with shortened venetoclax treatment cycles in patients with acute myeloid leukemia.Ann Hematol. 2024 Dec;103(12):6061. doi: 10.1007/s00277-024-06095-y. Ann Hematol. 2024. PMID: 39562361 Free PMC article. No abstract available.
Abstract
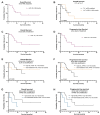
The combination of venetoclax with hypomethylating agents is currently the standard of care for elderly patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy. Despite its favorable efficacy, clinical use is often associated with post-remission cytopenia, frequently necessitating treatment delays and dose modifications. This study aims to evaluate the efficacy and safety of shortened venetoclax treatment durations. A multicenter analysis was conducted involving 20 adult AML patients receiving venetoclax (7 or 14 days with 9 and 11 patients, respectively) combined with 5-azacitidine (5-7 days) between 2021 and 2024. The cohort included patients from four German academic centers all treated in first line. Outcome measures included bone marrow response, transfusion dependence, overall survival (OS) and progression-free survival (PFS). Median age was 73.5 years, with 70% of patients having secondary AML. Adverse molecular risk was observed in 75% of patients. The overall response rate (ORR) was 100%, with a composite complete remission rate of 78%. No significant differences in response rates were observed between the 7-day and 14-day venetoclax regimens. Median OS for the cohort was 15 months. Infection-related complications were observed in 55% of patients, with severe sepsis in 20% of cases. In this cohort, shortened venetoclax regimens demonstrated efficacy comparable to standard treatment protocols, with a potential reduction in hematologic toxicity. These findings support the individualization of treatment regimens to optimize clinical outcomes while potentially minimizing adverse effects.
Keywords: 5-azacitidine; 7 + 7; Acute myeloid leukemia; Shortened treatment; Toxicity; Venetoclax.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The analysis was approved by the local ethics committee of Jena University Hospital, Germany (no. 3967–2/13 and 2024–3235) and of Würzburg University Hospital, Germany (no. 20240206 01). Competing interests: The authors declare no competing interests.
Figures


References
-
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Dohner H, Letai A, Fenaux P et al (2020) Azacitidine and Venetoclax in previously untreated Acute Myeloid Leukemia. N Engl J Med 383:617–629. 10.1056/NEJMoa2012971 - PubMed
-
- Pratz KW, DiNardo CD, Selleslag D, Li J, Yamamoto K, Konopleva M, McDonald A, Babu S, Stevens DA, Kantarjian HM et al (2020) Cytopenia Management in patients with newly diagnosed Acute myeloid leukemia treated with Venetoclax Plus Azacitidine in the VIALE-A study. Blood 136:51–53. 10.1182/blood-2020-134832
-
- Gross Z, Tauveron-Jalenques U, Aspas Requena G, Carre M, Meunier M, Tavernier E, Cornillon J, Belhabri A, Michallet M, Contejean A et al (2023) Real World Use of Azacitidine and Venetoclax in Acute myeloid leukemia in Frontline and Relapse/Refractory settings: a multicentric study from French Auraml Group. Blood 142:590–590. 10.1182/blood-2023-187331
-
- Zhu LX, Chen RR, Wang LL, Sun JN, Zhou D, Li L, Qian JJ, Zhang Y, Tong HY, Yu WJ et al (2022) A real-world study of infectious complications of venetoclax combined with decitabine or azacitidine in adult acute myeloid leukemia. Support Care Cancer 30:7031–7038. 10.1007/s00520-022-07126-y - PubMed
-
- Morsia E, McCullough K, Joshi M, Cook J, Alkhateeb HB, Al-Kali A, Begna K, Elliott M, Hogan W, Litzow M et al (2020) Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol 95:1511–1521. 10.1002/ajh.25978 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

