Design and development of dual targeting CAR protein for the development of CAR T-cell therapy against KRAS mutated pancreatic ductal adenocarcinoma using computational approaches
- PMID: 39453574
- PMCID: PMC11511808
- DOI: 10.1007/s12672-024-01455-6
Design and development of dual targeting CAR protein for the development of CAR T-cell therapy against KRAS mutated pancreatic ductal adenocarcinoma using computational approaches
Abstract
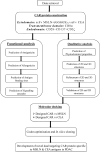
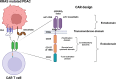
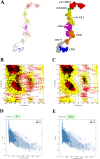
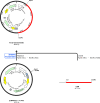
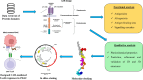
Mutant KRAS promotes the proliferation, metastasis, and aggressiveness of various cancers including pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), and colorectal adenocarcinoma (CRC) respectively. Mutant KRAS therapeutics are limited, while Sotorasib and Adagrasib were the only FDA-approved drugs for the treatment of KRASG12C mutated NSCLC. Chimeric antigen receptor (CAR) T-cell therapy has been emerged as an effective strategy against hematological malignancies and being extended towards solid cancers including PDAC. mesothelin (MSLN) and Carcinoembryonic Antigen (CEA) were reported to be highly overexpressed in KRAS-mutated PDAC. Meanwhile, in clinical trials, several CAR T-cell therapy studies are mainly focused towards these two cancer antigens in PDAC, however, the dual targeting of these two neoantigens is not reported. In the present study, we have designed and developed a novel dual-targeting CAR protein by employing various bioinformatics approaches such as functional analysis (antigenicity, allergenicity, antigen binding sites & signalling cascades), qualitative analysis (physicochemical, prediction, refinement & validation of 2D and 3D structures), molecular docking, and in silico cloning. Our results revealed that the designed CAR protein specifically binds with both MSLN & CEA with significant binding affinities, and was predicted to be stable & non-allergenic. Additionally, the protein-protein interaction network reveals the T-cell mediated antitumor responses of each domain in the designed CAR. Conclusively, we have designed and developed a dual targeting (MSLN & CEA) CAR protein towards KRAS-mutated PDAC using computational approaches. Alongside, we further recommend to engineer this designed CAR in T-cells and evaluating their therapeutic efficiency in in vitro and in vivo studies in the near future.
Keywords: CAR; CAR T-cell therapy; Immunotherapy; KRAS; Precision medicine; Therapeutics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
