Eligible Infants Included in Neonatal Clinical Trials and Reasons for Noninclusion: A Systematic Review
- PMID: 39453652
- PMCID: PMC11581680
- DOI: 10.1001/jamanetworkopen.2024.41372
Eligible Infants Included in Neonatal Clinical Trials and Reasons for Noninclusion: A Systematic Review
Abstract
Importance: Results of clinical trials can only represent included participants, and many neonatal trials fail due to insufficient participation. Infants not included in research may differ from those included in meaningful ways, biasing the sample and limiting the generalizability of findings.
Objective: To describe the proportion of eligible infants included in neonatal clinical trials and the reasons for noninclusion.
Evidence review: A systematic search of Cochrane CENTRAL was performed by retrieving articles meeting the following inclusion criteria: full-length, peer-reviewed articles describing clinical trial results in at least 20 human infants from US neonatal intensive care units, published in English, and added to Cochrane CENTRAL between 2017 and 2022. Retrieved articles were screened for inclusion by 2 independent researchers.
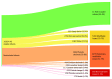
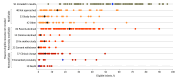
Findings: In total 120 articles met inclusion criteria and 91 of these (75.8%) reported the number of infants eligible for participation, which totaled 26 854 in aggregate. Drawing from these, an aggregate of 11 924 eligible infants (44.4%) were included in reported results. Among all eligible infants, most reasons for noninclusion in results were classified as modifiable or potentially modifiable by the research team. Parents declining to participate (8004 infants [29.8%]) or never being approached (2507 infants [9.3%]) were the 2 predominant reasons for noninclusion. Other modifiable reasons included factors related to study logistics, such as failure to appropriately collect data on enrolled infants (859 of 26 854 infants [3.2%]) and other reasons (1907 of 26 854 infants [7.1%]), such as loss to follow-up or eligible participants that were unaccounted for. Nonmodifiable reasons, including clinical change or death, accounted for a small proportion of eligible infants who were not included (858 of 26 854 infants [3.2%]).
Conclusions and relevance: This systematic review of reporting on eligible infants included and not included in neonatal clinical trials highlights the need for improved documentation on the flow of eligible infants through neonatal clinical trials and may also inform recruitment expectations for trialists designing future protocols. Improved adherence to standardized reporting may clarify which potential participants are being missed, improving understanding of the generalizability of research findings. Furthermore, these findings suggest that future work to understand why parents decline to participate in neonatal research trials and why some are never approached about research may help increase overall participation.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

