Effect of correcting iron deficiency on the risk of serious infection in heart failure: Insights from the IRONMAN trial
- PMID: 39453738
- PMCID: PMC11798631
- DOI: 10.1002/ejhf.3504
Effect of correcting iron deficiency on the risk of serious infection in heart failure: Insights from the IRONMAN trial
Abstract
Aims: Concerns exist that intravenous (IV) iron might increase the risk of infections. The IRONMAN trial provided an opportunity to investigate whether giving IV ferric derisomaltose (FDI) to patients with heart failure and iron deficiency alters the rate of hospitalization or death due to infections.
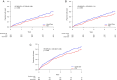
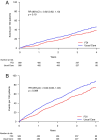
Methods and results: IRONMAN was a randomized trial of IV FDI versus usual care in patients with symptomatic heart failure, left ventricular ejection fraction (LVEF) ≤45%, and transferrin saturation (TSAT) <20% or ferritin <100 μg/L. Infection was a pre-specified, blindly-adjudicated, safety endpoint. The primary analysis of interest was infection as the main reason for hospitalization or death, using first and recurrent events analyses. The composite primary event of interest tended to be lower in those randomized to FDI when analysed as first (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.62-1.01, p = 0.055) or recurrent event (rate ratio 0.85, 95% CI 0.64-1.13, p = 0.089). The composite results were driven by fewer hospitalizations for infection (HR 0.76, 95% CI 0.49-0.98, p = 0.032), with 5% fewer patients (absolute reduction) experiencing such an event if assigned to FDI. Similar trends were observed for recurrent events (HR 0.82, 95% CI 0.62-1.10). Further analyses suggested that the reduction in hospitalizations due to infection with FDI was restricted to patients with TSAT <20%.
Conclusions: In patients with heart failure and a reduced LVEF, correction of iron deficiency is not associated with an increased risk of hospitalization or death from infection, and may reduce such events, especially when TSAT is <20%.
Clinical trial registration: ClinicalTrials.gov, NCT02642562.
Keywords: Heart failure; Hospitalization; Infection; Iron deficiency; Mortality.
© 2024 The Author(s). European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
References
-
- Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al.; AFFIRM‐AHF Investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double‐blind, randomised, controlled trial. Lancet 2020;396:1895–1904. 10.1016/S0140-6736(20)32339-4 - DOI - PubMed
-
- Kalra PR, Cleland JGF, Petrie MC, Thomson EA, Kalra PA, Squire IB, et al.; IRONMAN Study Group . Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): An investigator‐initiated, prospective, randomised, open‐label, blinded‐endpoint trial. Lancet 2022;400:2199–2209. 10.1016/S0140-6736(22)02083-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

