Biomechanics of phagocytosis of red blood cells by macrophages in the human spleen
- PMID: 39453740
- PMCID: PMC11536160
- DOI: 10.1073/pnas.2414437121
Biomechanics of phagocytosis of red blood cells by macrophages in the human spleen
Abstract
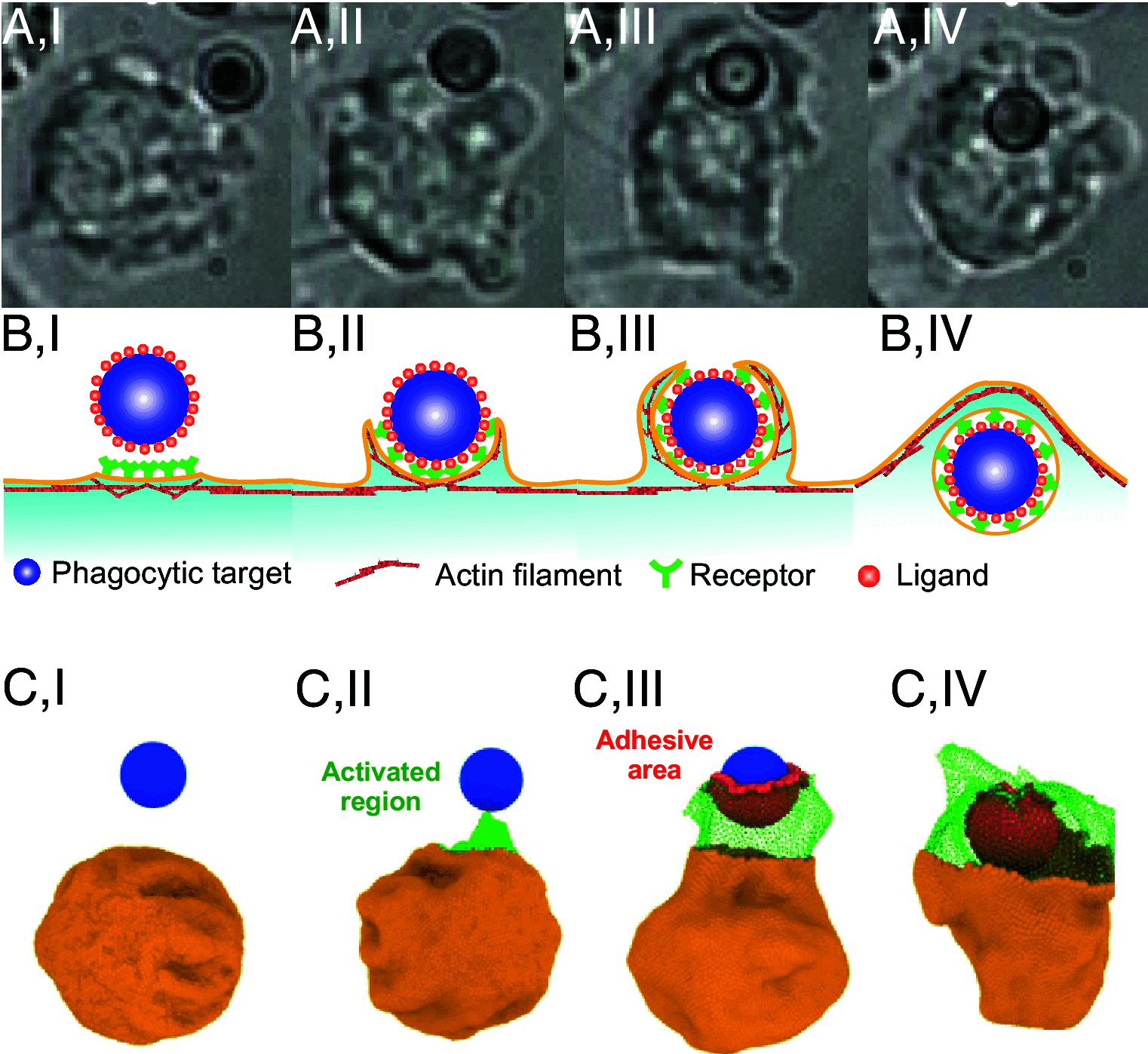
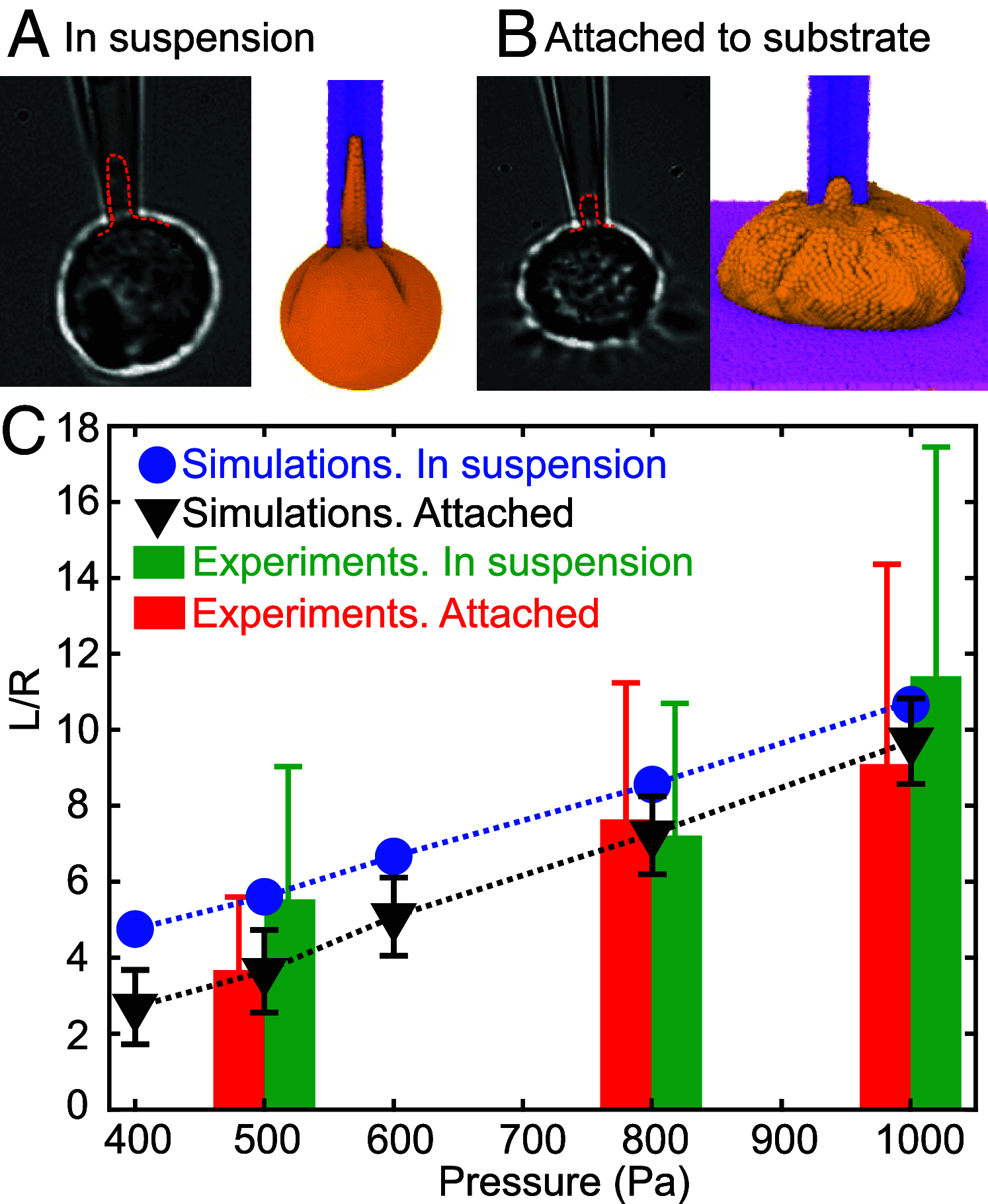
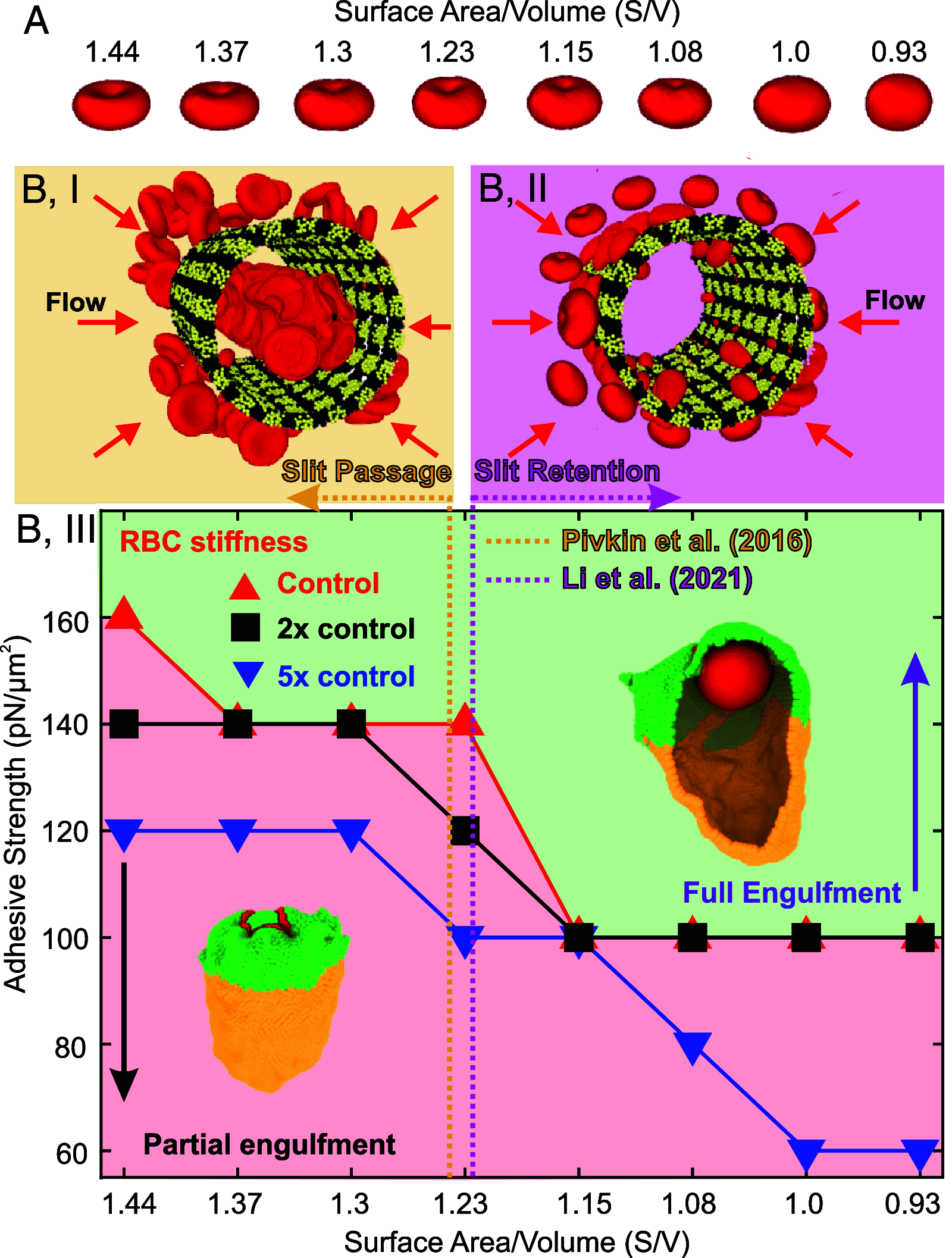
The clearance of senescent and altered red blood cells (RBCs) in the red pulp of the human spleen involves sequential processes of prefiltration, filtration, and postfiltration. While prior work has elucidated the mechanisms underlying the first two processes, biomechanical processes driving the postfiltration phagocytosis of RBCs retained at interendothelial slits (IES) are still poorly understood. We present here a unique computational model of macrophages to study the role of cell biomechanics in modulating the kinetics of phagocytosis of aged and diseased RBCs retained in the spleen. After validating the macrophage model using in vitro phagocytosis experiments, we employ it to probe the mechanisms underlying the kinetics of phagocytosis of mechanically altered RBCs, such as heated RBCs and abnormal RBCs in hereditary spherocytosis (HS) and sickle cell disease (SCD). Our simulations show pronounced deformation of the flexible and healthy RBCs in contrast to minimal shape changes in altered RBCs. Simulations also show that less deformable RBCs are engulfed faster and at lower adhesive strength than flexible RBCs, consistent with our experimental measurements. This efficient sensing and engulfment by macrophages of stiff RBCs retained at IES are expected to temper splenic congestion, a common pathogenic process in malaria, HS, and SCD. Altogether, our combined computational and in vitro experimental studies suggest that mechanical alterations of retained RBCs may suffice to enhance their phagocytosis, thereby adapting the kinetics of their elimination to the kinetics of their mechanical retention, an equilibrium essential for adequately cleaning the splenic filter to preserve its function.
Keywords: biomechanics; erythrophagocytosis; hypersplenism; macrophages; splenic sequestration.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Klei T., et al. , Hemolysis in the spleen drives erythrocyte turnover. Blood 136, 1579–1589 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

