Long-Term Effects of Empagliflozin in Patients with Chronic Kidney Disease
- PMID: 39453837
- PMCID: PMC7616743
- DOI: 10.1056/NEJMoa2409183
Long-Term Effects of Empagliflozin in Patients with Chronic Kidney Disease
Abstract
Background: In the EMPA-KIDNEY trial, empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, had positive cardiorenal effects in patients with chronic kidney disease who were at risk for disease progression. Post-trial follow-up was designed to assess how the effects of empagliflozin would evolve after the discontinuation of the trial drug.
Methods: In the active trial, patients with chronic kidney disease were randomly assigned to receive either empagliflozin (10 mg once daily) or matching placebo and were followed for a median of 2 years. All the patients had an estimated glomerular filtration rate (eGFR) of at least 20 but less than 45 ml per minute per 1.73 m2 of body-surface area or an eGFR of at least 45 but less than 90 ml per minute per 1.73 m2 with a urinary albumin-to-creatinine ratio (with albumin measured in milligrams and creatinine measured in grams) of at least 200. Subsequently, surviving patients who consented were observed for 2 additional years. No trial empagliflozin or placebo was administered during the post-trial period, but local practitioners could prescribe open-label SGLT2 inhibitors, including open-label empagliflozin. The primary composite outcome was kidney disease progression or cardiovascular death as assessed from the start of the active-trial period to the end of the post-trial period.
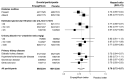
Results: Of the 6609 patients who had undergone randomization in the active trial, 4891 (74%) were enrolled in the post-trial period. During this period, the use of open-label SGLT2 inhibitors was similar in the two groups (43% in the empagliflozin group and 40% in the placebo group). During the combined active- and post-trial periods, a primary-outcome event occurred in 865 of 3304 patients (26.2%) in the empagliflozin group and in 1001 of 3305 patients (30.3%) in the placebo group (hazard ratio, 0.79; 95% confidence interval [CI], 0.72 to 0.87). During the post-trial period only, the hazard ratio for a primary-outcome event was 0.87 (95% CI, 0.76 to 0.99). During the combined periods, the risk of kidney disease progression was 23.5% in the empagliflozin group and 27.1% in the placebo group; the risk of the composite of death or end-stage kidney disease was 16.9% and 19.6%, respectively; and the risk of cardiovascular death was 3.8% and 4.9%, respectively. There was no effect of empagliflozin on death from noncardiovascular causes (5.3% in both groups).
Conclusions: In a broad range of patients with chronic kidney disease at risk for progression, empagliflozin continued to have additional cardiorenal benefits for up to 12 months after it was discontinued. (Funded by Boehringer Ingelheim and others; EMPA-KIDNEY ClinicalTrials.gov number, NCT03594110; EuDRACT number, 2017-002971-24.).
Copyright © 2024 Massachusetts Medical Society.
Figures

Comment in
-
Long-Term Effects of Empagliflozin in Chronic Kidney Disease.N Engl J Med. 2025 Jun 12;392(22):2284-2285. doi: 10.1056/NEJMc2504181. N Engl J Med. 2025. PMID: 40499178 No abstract available.
-
Long-Term Effects of Empagliflozin in Chronic Kidney Disease.N Engl J Med. 2025 Jun 12;392(22):2285. doi: 10.1056/NEJMc2504181. N Engl J Med. 2025. PMID: 40499179 No abstract available.
-
Long-Term Effects of Empagliflozin in Chronic Kidney Disease. Reply.N Engl J Med. 2025 Jun 12;392(22):2285-2286. doi: 10.1056/NEJMc2504181. N Engl J Med. 2025. PMID: 40499180 No abstract available.
References
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389(10075):1238–1252. - PubMed
-
- Renal Studies Group Nuffield Department of Population Health Renal Studies and SMART-Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8. - DOI - PMC - PubMed
-
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
