Mitochondrial Bioenergetics of Functional Wound Closure is Dependent on Macrophage-Keratinocyte Exosomal Crosstalk
- PMID: 39453865
- PMCID: PMC11544725
- DOI: 10.1021/acsnano.4c07610
Mitochondrial Bioenergetics of Functional Wound Closure is Dependent on Macrophage-Keratinocyte Exosomal Crosstalk
Abstract
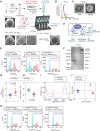
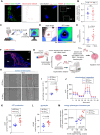
Tissue nanotransfection (TNT)-based fluorescent labeling of cell-specific exosomes has shown that exosomes play a central role in physiological keratinocyte-macrophage (mϕ) crosstalk at the wound-site. Here, we report that during the early phase of wound reepithelialization, macrophage-derived exosomes (Exomϕ), enriched with the outer mitochondrial membrane protein TOMM70, are localized in leading-edge keratinocytes. TOMM70 is a 70 kDa adaptor protein anchored in the mitochondrial outer membrane and plays a critical role in maintaining mitochondrial function and quality. TOMM70 selectively recognizes cytosolic chaperones by its tetratricopeptide repeat (TPR) domain and facilitates the import of preproteins lacking a positively charged mitochondrial targeted sequence. Exosomal packaging of TOMM70 in mϕ was independent of mitochondrial fission. TOMM70-enriched Exomϕ compensated for the hypoxia-induced depletion of epidermal TOMM70, thereby rescuing mitochondrial metabolism in leading-edge keratinocytes. Thus, macrophage-derived TOMM70 is responsible for the glycolytic ATP supply to power keratinocyte migration. Blockade of exosomal uptake from keratinocytes impaired wound closure with the persistence of proinflammatory mϕ in the wound microenvironment, pointing toward a bidirectional crosstalk between these two cell types. The significance of such bidirectional crosstalk was established by the observation that in patients with nonhealing diabetic foot ulcers, TOMM70 is deficient in keratinocytes of wound-edge tissues.
Keywords: TOMM70; functional wound closure; keratinocyte migration; macrophage-derived exosomes; macrophage–keratinocyte crosstalk; tissue nanotransfection; “don’t eat me” plasmid.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

