A prospective Real-Life Multicenter Study of Tildrakizumab 200 mg in Patients with Moderate-Severe Psoriasis: Who is the Ideal Patient?
- PMID: 39453881
- PMCID: PMC11620192
- DOI: 10.5826/dpc.1404a284
A prospective Real-Life Multicenter Study of Tildrakizumab 200 mg in Patients with Moderate-Severe Psoriasis: Who is the Ideal Patient?
Abstract
Introduction: Tildrakizumab, a humanized monoclonal antibody targeting the p19 subunit of interleukin 23 (IL-23), has shown promise in the management of moderate-to-severe plaque psoriasis, offering potential improvements in clinical outcomes and quality of life.
Objectives: The study aimed to identify patient characteristics that indicate the initiation of a 200 mg dosage of tildrakizumab in a real-world setting, focusing on factors that enhance treatment effectiveness and safety.
Methods: This prospective study included 54 adult patients with moderate-to-severe plaque psoriasis treated with tildrakizumab 200 mg from March 2023 to March 2024 across 13 Italian Dermatology Units. Data collected included demographics, disease duration, comorbidities, and previous treatments. PASI, BSA, and DLQI scores were recorded at baseline and at weeks 4, 16, and 28. Safety was assessed through adverse event reporting. Univariate analysis was performed to identify baseline characteristics significantly associated with achieving PASI ≤ 5 at week 16.
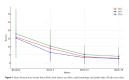
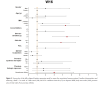
Results: Significant reductions in PASI scores were observed at week 4 (9 ± 6.9, P < 0.001), with further improvements at weeks 16 (3.9 ± 4.2, P < 0.001) and 28 (2.9 ± 4.4, P < 0.001). Univariate analysis showed that obese patients (BMI > 30) had higher odds (OR = 4.333, P < 0.05) of achieving PASI ≤ 5. Longer disease duration and starting with a 100 mg dosage also correlated with better outcomes. The safety profile was favorable, with minimal adverse events reported.
Conclusions: Tildrakizumab 200 mg is effective and safe for moderate-to-severe psoriasis, particularly in obese patients. These findings support its use as a long-term treatment option.
Conflict of interest statement
Figures
References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013 Feb;133(2):377–85. doi: 10.1038/jid.2012.339. Epub 2012 Sep 27. - DOI - PubMed
LinkOut - more resources
Full Text Sources
