Capillary drops, capillary pooled, and venous blood samples for determining hemoglobin concentration using HemoCue: A measurement system analysis
- PMID: 39453907
- PMCID: PMC11508077
- DOI: 10.1371/journal.pone.0312233
Capillary drops, capillary pooled, and venous blood samples for determining hemoglobin concentration using HemoCue: A measurement system analysis
Abstract
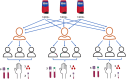
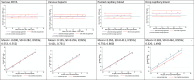
Several external and internal factors can affect the performance and variability of Hemoglobin concentration [Hb] measurements using HemoCue, and documentation on the contribution of different sources of [Hb] variation is limited. We used an experimental repeated measurements design with nine randomly selected participants, three HemoCue devices, and three trained field workers. HemoCue measurements for all samples were repeated three times. The [Hb] measurement system considers four sources of error: 1) HemoCue devices, 2) field workers, 3) between individuals, and 4) within individuals. A concordance analysis was used to assess accuracy and precision, and a linear mixed model was used to estimate mean differences (bias) from blood specimens, anticoagulants, and to estimate the contribution of the 4 sources of error to [Hb] measurements. Positive mean [Hb] differences were found: 1.34 g/dL for capillary drops, 0.81 g/dL for pooled capillary blood samples, 0.756 g/dL for venous blood stored with EDTA, and 0.911 g/dL for venous blood stored with heparin. The mean [Hb] difference for venous blood with EDTA was used as a correction factor for all results measured using a HemoCue. After adjustment, capillary drops showed a mean difference of 0.585 g/dL, and pooled capillary samples were not significantly different. The individual variability was 95.8% of total variance, HemoCue devices contributed 2.1% of measurement error, field staff contributed 0.4%, and the residual was 1.7%. The HemoCue [Hb] measurement system is reliable in controlled environments, with a small measurement error of 4.2%.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
OD works for USAID but acted in his personal capacity; the content of this article does not necessarily reflect the views of USAID or the United States government.
Figures
References
-
- Stevens GA, Paciorek CJ, Flores-Urrutia MC, Borghi E, Namaste S, Wirth JP, et al. National, Regional, and Global Estimates of Anaemia by Severity in Women and Children for 2000–19: A Pooled Analysis of Population-Representative Data. Lancet Glob Health. 2022. May;10(5):E627–E639. doi: 10.1016/S2214-109X(22)00084-5 ; PMCID: PMC9023869. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

