The mechanism of NF-κB-TERT feedback regulation of granulosa cell apoptosis in PCOS rats
- PMID: 39453929
- PMCID: PMC11508119
- DOI: 10.1371/journal.pone.0312115
The mechanism of NF-κB-TERT feedback regulation of granulosa cell apoptosis in PCOS rats
Abstract
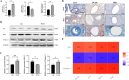
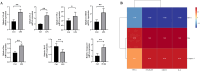
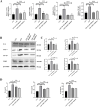
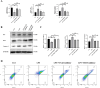
Patients with Polycystic ovary syndrome (PCOS) have chronic low-grade ovarian inflammation. Inflammation can cause telomere dysfunction, and telomere and telomerase complex are also involved in regulating inflammation. However, the specific mechanisms of inflammatory signaling feedback and telomere-telomerase mutual regulation remain to be discovered. This study elucidates the role of Nuclear factor kappa-B (NF-κB)-Telomerase reverse transcriptase (TERT) feedback in PCOS granulosa cell apoptosis. Using letrozole and a high-fat diet, a PCOS rat model was established, along with a Lipopolysaccharide (LPS) -treated KGN cell inflammation model was established. NF-κB and TERT inhibitors (BAY 11-7082 and BIBR1532) were then administered to LPS-induced KGN cells. PCOS rats displayed disrupted estrous cycles, increased weight, elevated serum testosterone, cystic follicles, granulosa cell layer thinning, and reduced corpora lutea count (P are all less than 0.05). In PCOS rat ovaries, NF-κB, Interleukin-6 (IL-6), Tumor Necrosis Factor α (TNF-α), TERT, Bax, and Caspase-3 exhibited notable upregulation, while Bcl-2 decreased, with telomere elongation (P are all less than 0.05). There were significant correlations among NF-κB-related inflammatory factors, TERT and apoptotic factors, and they were positively correlated with Bax and Caspase-3, and negatively correlated with Bcl-2 (P are all less than 0.05). LPS-treated KGN cells demonstrated increased expression of inflammatory and pro-apoptotic factors, later restored post-treatment with NF-κB and TERT inhibitors (P are all less than 0.05). In conclusion, TERT may induce granulosa cell apoptosis by participating in the regulation of the NF-κB signaling pathway, thereby mediating the chronic inflammatory response of PCOS through downstream inflammatory factors IL-6 and TNF-α.
Copyright: © 2024 Xue et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Ghrelin Alleviates Inflammation, Insulin Resistance, and Reproductive Abnormalities in Mice with Polycystic Ovary Syndrome via the TLR4-NF-κB Signaling Pathway.Discov Med. 2024 May;36(184):946-958. doi: 10.24976/Discov.Med.202436184.88. Discov Med. 2024. PMID: 38798254
-
Serum granulosa cell-derived TNF-α promotes inflammation and apoptosis of renal tubular cells and PCOS-related kidney injury through NF-κB signaling.Acta Pharmacol Sin. 2023 Dec;44(12):2432-2444. doi: 10.1038/s41401-023-01128-0. Epub 2023 Jul 28. Acta Pharmacol Sin. 2023. PMID: 37507430 Free PMC article.
-
YKL-40 Knockdown Decreases Oxidative Stress Damage in Ovarian Granulosa Cells.Genet Test Mol Biomarkers. 2024 May;28(5):199-206. doi: 10.1089/gtmb.2023.0361. Epub 2024 Apr 18. Genet Test Mol Biomarkers. 2024. PMID: 38634621
-
Chlorogenic acid mitigates DHEA-induced oxidative stress in granulosa cells and alleviates ferroptosis via the NF-κB signaling pathway in PCOS.Eur J Pharmacol. 2025 Sep 5;1002:177870. doi: 10.1016/j.ejphar.2025.177870. Epub 2025 Jun 20. Eur J Pharmacol. 2025. PMID: 40544934
-
The Novel Insight of Gut Microbiota from Mouse Model to Clinical Patients and the Role of NF-κB Pathway in Polycystic Ovary Syndrome.Reprod Sci. 2024 Nov;31(11):3323-3333. doi: 10.1007/s43032-024-01562-3. Epub 2024 Apr 23. Reprod Sci. 2024. PMID: 38653859 Review.
Cited by
-
Dysregulation of autophagy during photoaging reduce oxidative stress and inflammatory damage caused by UV.Front Pharmacol. 2025 May 12;16:1562845. doi: 10.3389/fphar.2025.1562845. eCollection 2025. Front Pharmacol. 2025. PMID: 40421222 Free PMC article. Review.
-
Analysis of TERT mRNA Levels and Clinicopathological Features in Patients with Peritoneal Mesothelioma.Cancers (Basel). 2025 Jan 14;17(2):252. doi: 10.3390/cancers17020252. Cancers (Basel). 2025. PMID: 39858033 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

