Mice lacking acid-sensing ion channel 2 in the medial prefrontal cortex exhibit social dominance
- PMID: 39453995
- PMCID: PMC11506137
- DOI: 10.1126/sciadv.adn7573
Mice lacking acid-sensing ion channel 2 in the medial prefrontal cortex exhibit social dominance
Abstract
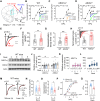
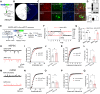
Social dominance is essential for maintaining a stable society and has both positive and negative impacts on social animals, including humans. However, the regulatory mechanisms governing social dominance, as well as the crucial regulators and biomarkers involved, remain poorly understood. We discover that mice lacking acid-sensing ion channel 2 (ASIC2) exhibit persistently higher social dominance than their wild-type cagemates. Conversely, overexpression of ASIC2 in the medial prefrontal cortex reverses the dominance hierarchy observed in ASIC2 knockout (Asic2-/-) mice. Asic2-/- neurons exhibit increased synaptic transmission and plasticity, potentially mediated by protein kinase A signaling pathway. Furthermore, ASIC2 plays distinct functional roles in excitatory and inhibitory neurons, thereby modulating the balance of neuronal activities underlying social dominance behaviors-a phenomenon suggestive of a cell subtype-specific mechanism. This research lays the groundwork for understanding the mechanisms of social dominance, offering potential insights for managing social disorders, such as depression and anxiety.
Figures






References
-
- Baum A., Garofalo J. P., Yali A. M., Socioeconomic status and chronic stress: Does stress account for SES effects on health? Ann. N. Y. Acad. Sci. 896, 131–144 (1999). - PubMed
-
- Ennals R., Michael Marmot (2004) Status Syndrome: How your social standing directly affects your health and life expectancy. AI Soc. 21, 231–233 (2006).
-
- Bronson F. H., The reproductive ecology of the house mouse. Q. Rev. Biol. 54, 265–299 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

