Toxoplasma gondii macrophage migration inhibitory factor shows anti- Mycobacterium tuberculosis potential via AZIN1/STAT1 interaction
- PMID: 39453997
- PMCID: PMC11506136
- DOI: 10.1126/sciadv.adq0101
Toxoplasma gondii macrophage migration inhibitory factor shows anti- Mycobacterium tuberculosis potential via AZIN1/STAT1 interaction
Abstract
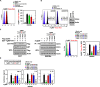
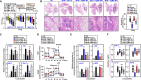
Mycobacterium tuberculosis (MTB) is a pathogenic bacterium, belonging to the family Mycobacteriaceae, that causes tuberculosis (TB). Toxoplasma gondii macrophage migration inhibitory factor (TgMIF), a protein homolog of macrophage migration inhibitory factor, has been explored for its potential to modulate immune responses during MTB infections. We observed that TgMIF that interacts with CD74, antizyme inhibitor 1 (AZIN1), and signal transducer and activator of transcription 1 (STAT1) modulates endocytosis, restoration of mitochondrial function, and macrophage polarization, respectively. These interactions promote therapeutic efficacy in mice infected with MTB, thereby presenting a potential route to host-directed therapy development. Furthermore, TgMIF, in combination with first-line TB drugs, significantly inhibited drug-resistant MTB strains, including multidrug-resistant TB. These results demonstrate that TgMIF is potentially a multifaceted therapeutic agent against TB, acting through immune modulation, enhancement of mitochondrial function, and dependent on STAT1 and AZIN1 pathways.
Figures






References
-
- Singh R., Dwivedi S. P., Gaharwar U. S., Meena R., Rajamani P., Prasad T., Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 128, 1547–1567 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

