Accelerated Alzheimer's Aβ-42 secondary nucleation chronologically visualized on fibril surfaces
- PMID: 39454002
- PMCID: PMC11506133
- DOI: 10.1126/sciadv.adp5059
Accelerated Alzheimer's Aβ-42 secondary nucleation chronologically visualized on fibril surfaces
Abstract
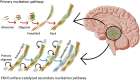
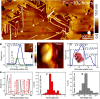
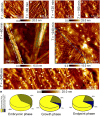
Protein fibril surfaces tend to generate toxic oligomers catalytically. To date, efforts to study the accelerated aggregation steps involved with Alzheimer's disease-linked amyloid-β (Aβ)-42 proteins on fibril surfaces have mainly relied on fluorophore-based analytics. Here, we visualize rare secondary nucleation events on the surface of Aβ-42 fibrils from embryonic to endpoint stages using liquid-based atomic force microscopy. Nanoscale imaging supported by atomic-scale molecular simulations tracked the adsorption and proliferation of oligomeric assemblies at nonperiodically spaced catalytic sites on the fibril surface. Upon confirming that fibril edges are preferential binding sites for oligomers during embryonic stages, the secondary fibrillar size changes were quantified during the growth stages. Notably, a small population of fibrils that displayed higher surface catalytic activity was identified as superspreaders. Profiling secondary fibrils during endpoint stages revealed a nearly threefold increase in their surface corrugation, a parameter we exploit to classify fibril subpopulations.
Figures




Similar articles
-
Off-pathway oligomers of α-synuclein and Aβ inhibit secondary nucleation of α-synuclein amyloid fibrils.J Mol Biol. 2025 May 15;437(10):169048. doi: 10.1016/j.jmb.2025.169048. Epub 2025 Feb 25. J Mol Biol. 2025. PMID: 40015369
-
Protofibril-Fibril Interactions Inhibit Amyloid Fibril Assembly by Obstructing Secondary Nucleation.Angew Chem Int Ed Engl. 2021 Feb 8;60(6):3016-3021. doi: 10.1002/anie.202010098. Epub 2020 Dec 11. Angew Chem Int Ed Engl. 2021. PMID: 33095508 Free PMC article.
-
Understanding Amyloid-β Oligomerization at the Molecular Level: The Role of the Fibril Surface.Chemistry. 2016 Jun 20;22(26):8768-72. doi: 10.1002/chem.201601701. Epub 2016 May 24. Chemistry. 2016. PMID: 27135646
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
Alzheimer's beta-amyloid: insights into fibril formation and structure from Congo red binding.Subcell Biochem. 2005;38:203-24. doi: 10.1007/0-387-23226-5_10. Subcell Biochem. 2005. PMID: 15709480 Review.
Cited by
-
On Levodopa Interactions with Brain Disease Amyloidogenic Proteins at the Nanoscale.ACS Omega. 2025 Apr 2;10(14):14487-14495. doi: 10.1021/acsomega.5c01028. eCollection 2025 Apr 15. ACS Omega. 2025. PMID: 40256523 Free PMC article.
References
-
- Maccioni R. B., Muñoz J. P., Barbeito L., The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 32, 367–381 (2001). - PubMed
-
- Vergallo A., Mégret L., Lista S., Cavedo E., Zetterberg H., Blennow K., Vanmechelen E., de Vos A., Habert M.-O., Potier M.-C., Dubois B., Neri C., Hampel H.; INSIGHT-preAD study group; Alzheimer Precision Medicine Initiative (APMI) , Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement. 15, 764–775 (2019). - PubMed
-
- Schindler S. E., Bollinger J. G., Ovod V., Mawuenyega K. G., Li Y., Gordon B. A., Holtzman D. M., Morris J. C., Benzinger T. L. S., Xiong C., Fagan A. M., Bateman R. J., High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

