Coiled Coil Peptide Tiles (CCPTs): Expanding the Peptide Building Block Design with Multivalent Peptide Macrocycles
- PMID: 39454098
- PMCID: PMC11544620
- DOI: 10.1021/jacs.4c09531
Coiled Coil Peptide Tiles (CCPTs): Expanding the Peptide Building Block Design with Multivalent Peptide Macrocycles
Abstract
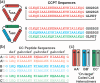
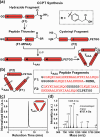
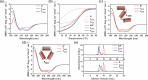
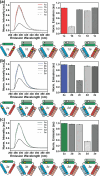
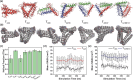
Owing to their synthetic accessibility and protein-mimetic features, peptides represent an attractive biomolecular building block for the fabrication of artificial biomimetic materials with emergent properties and functions. Here, we expand the peptide building block design space through unveiling the design, synthesis, and characterization of novel, multivalent peptide macrocycles (96mers), termed coiled coil peptide tiles (CCPTs). CCPTs comprise multiple orthogonal coiled coil peptide domains that are separated by flexible linkers. The constraints, imposed by cyclization, confer CCPTs with the ability to direct programmable, multidirectional interactions between coiled coil-forming "edge" domains of CCPTs and their free peptide binding partners. These fully synthetic constructs are assembled using a convergent synthetic strategy via a combination of native chemical ligation and Sortase A-mediated cyclization. Circular dichroism (CD) studies reveal the increased helical stability associated with cyclization and subsequent coiled coil formation along the CCPT edges. Size-exclusion chromatography (SEC), analytical high-performance liquid chromatography (HPLC), and fluorescence quenching assays provide a comprehensive biophysical characterization of various assembled CCPT complexes and confirm the orthogonal colocalization between coiled coil domains within CCPTs and their designed on-target free peptide partners. Lastly, we employ molecular dynamics (MD) simulations, which provide molecular-level insights into experimental results, as a supporting method for understanding the structural dynamics of CCPTs and their complexes. MD analysis of the simulated CCPT architectures reveals the rigidification and expansion of CCPTs upon complexation, i.e., coiled coil formation with their designed binding partners, and provides insights for guiding the designs of future generations of CCPTs. The addition of CCPTs into the repertoire of coiled coil-based building blocks has the potential for expanding the coiled coil assembly landscape by unlocking new topologies having designable intermolecular interfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Levin A.; Hakala T. A.; Schnaider L.; Bernardes G. J. L.; Gazit E.; Knowles T. P. J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4 (11), 615–634. 10.1038/s41570-020-0215-y. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

