Gene Therapy for Inflammatory Cascade in Intrauterine Injury with Engineered Extracellular Vesicles Hybrid Snail Mucus-enhanced Adhesive Hydrogels
- PMID: 39454114
- PMCID: PMC11714243
- DOI: 10.1002/advs.202410769
Gene Therapy for Inflammatory Cascade in Intrauterine Injury with Engineered Extracellular Vesicles Hybrid Snail Mucus-enhanced Adhesive Hydrogels
Abstract
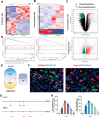
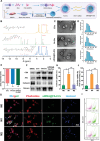
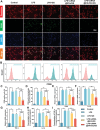
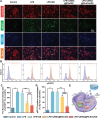
Early hyper-inflammation caused by intrauterine injury triggered subsequent intrauterine adhesion (IUA). STAT1-mediated M1 macrophages are confirmed to secrete pro-inflammatory cytokines to accelerate inflammatory cascade and IUA formation by multi-omics analysis and experimental verification. However, clinically used hyaluronic acid (HA) hydrogels are prone to slip out of injury sites due to poor bio-adhesion properties. Therefore, there are still challenges in applying hydrogels for M1 macrophage intervention in IUA treatment. Herein, an engineered extracellular vesicles (EVs) hybrid snail mucus (SM)-enhanced adhesive hydrogels to improve bio-adhesion property is fabricated and M1 macrophage intervention through targeting delivery and STAT1 silencing is achieved. First, inspired by the high bio-adhesion capacity of SM, SM and gelatin methacrylate (GelMA) solution are mixed to construct GelMA/SM (GS) hydrogel. Then, folic acid-modified extracellular vesicles (FA-EVs) are synthesized for targeting the delivery of STAT1-siRNA. Upon injection of FA-EVs hybrid GS hydrogel into the uterine cavity, a protective hydrogel layer forms on the surface of injury sites and sustains the release of STAT1-siRNA-loaded FA-EVs to curtail M1 macrophages generation through inhibiting STAT1 phosphorylation, resulting in reduction of myofibroblasts activation and collagen deposition. In addition, the pregnancy rate and the number of fetuses in rats treated with this hydrogel were much higher than those in other groups, suggesting that the hydrogel could promote functional endometrial regeneration and restore fertility. Overall, this study presents a promising strategy for employing FA-EVs hybrid adhesive hydrogel with superior bio-adhesion properties and M1 macrophage targeting delivery for IUA treatment and uterus recovery.
Keywords: adhesive hydrogels; extracellular vesicles; gene therapy; intrauterine adhesions; macrophage polarization.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Thermosensitive hydrogel as a sustained release carrier for mesenchymal stem cell-derived extracellular vesicles in the treatment of intrauterine adhesion.J Nanobiotechnology. 2024 Sep 17;22(1):570. doi: 10.1186/s12951-024-02780-2. J Nanobiotechnology. 2024. PMID: 39289737 Free PMC article.
-
Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels-Alder click reaction for the treatment of intrauterine adhesions.Acta Biomater. 2024 Mar 15;177:77-90. doi: 10.1016/j.actbio.2024.02.001. Epub 2024 Feb 6. Acta Biomater. 2024. PMID: 38331133
-
Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment.Eur J Pharm Sci. 2020 May 30;148:105316. doi: 10.1016/j.ejps.2020.105316. Epub 2020 Mar 19. Eur J Pharm Sci. 2020. PMID: 32201342
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Multifunctional hydrogel-based engineered extracellular vesicles delivery for complicated wound healing.Theranostics. 2024 Jul 8;14(11):4198-4217. doi: 10.7150/thno.97317. eCollection 2024. Theranostics. 2024. PMID: 39113809 Free PMC article. Review.
Cited by
-
Extracellular vesicles from adipose-derived stromal/stem cells reprogram dendritic cells to alleviate rat TMJOA by transferring mitochondria.J Nanobiotechnology. 2025 May 28;23(1):389. doi: 10.1186/s12951-025-03478-9. J Nanobiotechnology. 2025. PMID: 40426246 Free PMC article.
-
Nature-derived microneedles with metal-polyphenolic networks encapsulation for chronic soft tissue defects repair: Responding and remodeling the regenerative microenvironment.Mater Today Bio. 2025 Feb 1;31:101539. doi: 10.1016/j.mtbio.2025.101539. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40026624 Free PMC article.
References
-
- a) Peng X., Zhu Y., Wang T., Wang S., Sun J., Aging 2023, 15, 8275; - PMC - PubMed
- b) Wu F., Lei N., Yang S., Zhou J., Chen M., Chen C., Qiu L., Guo R., Li Y., Chang L., Front. Bioeng. Biotechnol. 2023, 11, 1264006; - PMC - PubMed
- c) Kou L., Jiang X., Xiao S., Zhao Y. Z., Yao Q., Chen R., J. Control. Rel. 2020, 318, 25. - PubMed
-
- Yi X., Liu F., Gao K., Chen F., Wang Y., Li H., Wang X., Huang Y., Fu H., Zhou W., Fan J. B., Wang S., Gao Y., Adv. Mater. 2022, 34, 2106510. - PubMed
-
- Yu‐Hung L., Tsrang‐Neng J., Jiann‐Loung H., Lee‐Wen H., Kok‐Min S., Bih‐Chwen H., Chien‐Hsien H., Fertil. Steril. 2015, 103, 513. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
