Long-term real-world evidence of CPX-351 of high-risk patients with AML identified high rate of negative MRD and prolonged OS
- PMID: 39454204
- PMCID: PMC11869862
- DOI: 10.1182/bloodadvances.2024014279
Long-term real-world evidence of CPX-351 of high-risk patients with AML identified high rate of negative MRD and prolonged OS
Abstract
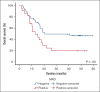
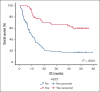
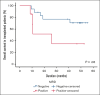
CPX-351 has been approved for patients with therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (MRC-AML). No extensive data are available on measurable residual disease (MRD) and long-term clinical outcome using CPX-351 in AML in real life. We retrospectively collected data from 168 patients in 36 centers in France and Italy who had received 1 or 2 cycles of induction with CPX-351. All patients were aged >18 years and had newly diagnosed, untreated t-AML or MRC-AML. With a median follow-up of 3 years, the median overall survival (OS) was 13.3 months. The median OS was 20.4 months vs 12.9 months for patients with MRD below or above 10-3, respectively (P = .006). In a multivariate analysis, only MRD >10-3 was associated with a poorer OS (hazard ratio, 2.6; 95% confidence interval, 1.2-5.5; P = .013). We also observed a trend toward a better median OS in patients who underwent hematopoietic stem cell transplantation with MRD <10-3 (not reached vs 26.0 months; P = .06). Achievement of MRD negativity contributed to the improvement of OS in the overall population and, maybe, in patients receiving transplant. These data provide the rationale for the 2 ongoing studies evaluating CPX-351 vs 7+3 in non-MRC-AML and non-t-AML using MRD as the primary end point for ALFA-2101 phase 2 clinical trial and event-free survival for AMLSG 30-18 phase 3 clinical trial.
© 2025 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.C., R.M.L., and L.A. declare consultancy and speaker bureau fees from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures
References
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. - PubMed
-
- Roboz GJ, Larson ML, Rubenstein SE, et al. Final safety and efficacy results from the CPX-351 early access program for older patients with high-risk or secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(5):1188–1194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources