Ibrutinib for therapy of steroid-refractory chronic graft-versus-host disease: a multicenter real-world analysis
- PMID: 39454280
- PMCID: PMC11909441
- DOI: 10.1182/bloodadvances.2024014374
Ibrutinib for therapy of steroid-refractory chronic graft-versus-host disease: a multicenter real-world analysis
Abstract
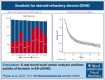
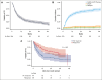
To examine the activity of ibrutinib in steroid-refractory chronic graft-versus-host disease (SR-cGVHD) after the US Food and Drug Administration approval, we conducted a multicenter retrospective study. Data were standardly collected (N = 270 from 19 centers). Involved organs included skin (75%), eye (61%), mouth (54%), joint/fascia (47%), gastrointestinal (GI) (26%), lung (27%), liver (19%), genital (7%), and others (4.4%). The National Institutes of Health (NIH) severity was mild in 5.7%, moderate 42%, and severe 53%. Thirty-nine percent had overlap subtype. Karnofsky performance status (KPS) was ≥80% in 72%. The median prednisone was 0.21 mg/kg (0-2.27). Ibrutinib was started at a median of 18.2 months after cGVHD onset and in earlier lines of therapy (second line, 26%; third, 30%; fourth, 21%; fifth, 9.6%; sixth, 10%; seventh or higher, 1.2%). Among evaluable patients, the 6-month NIH overall response rate (ORR; complete response [CR]/partial response [PR]) was 45% (PR 42%; CR 3%). The median duration of response was 15 months (range, 1-46). Liver involvement had association with 6-month ORR (multivariate [MVA] odds ratio, 5.49; 95% confidence interval [CI], 2.3-14.2; P < .001). The best overall response was 56%, with most (86%) achieving by 1 to 3 months. With a median follow-up for survivors of 30.5 months, failure-free survival (FFS) was 59% (53%-65%) at 6 months and 41% (36%-48%) at 12 months. On MVA, increased age (hazard ratio [HR], 1.01; 95% CI, 1.0-1.02; P = .033), higher baseline prednisone (HR, 1.92; 95% CI, 1.09-3.38; P = .032), and lung involvement (HR, 1.58; 95% CI, 1.1-2.28; P = .016) had worse FFS. Ibrutinib discontinuation was most commonly due to progressive cGVHD (44%) or toxicity (42%). These data support that ibrutinib has activity in SR-cGVHD, provide new insight into factors associated with response and FFS, and demonstrate the toxicity profile associated with discontinuation.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.P. reports consulting and advisory board membership fees from Syndax, CTI BioPharma, Amgen, Regeneron, and Incyte; and clinical trial support from Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, CTI BioPharma, and Bristol Myers Squibb. M.E.D.F. reports research funding from Pharmacyclics, Novartis, and Incyte. Z.D. reports research support from Incyte Corp, REGiMMUNE Corp, and Taiho Oncology, Inc; consulting fees from Sanofi, Incyte Corp, Inhibrx, PharmaBiome AG, Ono Pharmaceutical, REGiMMUNE Corp, MaaT Pharma, and Forte Biosciences Inc. I.P. reports advisory board membership fees from Incyte, Sanofi, and Syndax. M.Q. reports consultancy fees from Novartis and Vertex. A.S. reports research funding from Merck and Novartis; research product support from Clasado, DSM/iHealth, and Blue Spark Technologies; and consulting fees from TargaZyme, Acrotech, Geron, and Janssen. H.R. serves as the medical monitor for the National Marrow Donor Program CD33 CAR-T trial; and reports advisory board fees from Medexus Pharmaceuticals. W.H. reports advisory board membership fees from Nkarta, Sanofi, Incyte, Rigel, and MaaT Pharma; consultancy fees from ACI Group and Therakos/Mallinckrodt; data and safety monitoring board membership fees from Angiocrine; and adjudication committee fees from CSL Behring. M.H. reports research funding from ADC Therapeutics, Spectrum Pharmaceuticals, and Astellas; consultancy fees from ADC Therapeutics, CRISPR, Bristol Myers Squibb, Kite, AbbVie, Caribou, Genmab, Autolus, and Forte Biosciences; and speaker's bureau fees from ADC Therapeutics, AstraZeneca, BeiGene, Kite, DMC Inc, Genentech, Myeloid Therapeutics, and CRISPR. N.F. reports advisory board and speaker fees from Incyte; DSMB membership fees from Chronic GVHD Consortium; and medical monitor duties Blood and Marrow Transplant Clinical Trials Network. C.K. reports consultancy fees from Incyte and Horizon Therapeutics; and advisory board fees from Incyte. E.N. reports consultancy fees from Medexus Pharmaceuticals and Novartis. The remaining authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials