TFEB agonist clomiphene citrate activates the autophagy-lysosomal pathway and ameliorates Alzheimer's disease symptoms in mice
- PMID: 39454957
- PMCID: PMC11599454
- DOI: 10.1016/j.jbc.2024.107929
TFEB agonist clomiphene citrate activates the autophagy-lysosomal pathway and ameliorates Alzheimer's disease symptoms in mice
Abstract
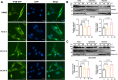
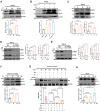
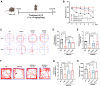
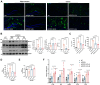
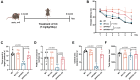
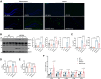
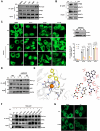
Autophagy is a conserved eukaryotic cellular clearance and recycling process through the lysosome-mediated degradation of damaged organelles and protein aggregates to maintain homeostasis. Impairment of the autophagy-lysosomal pathway is implicated in the pathogenesis of Alzheimer's disease (AD). Transcription factor EB (TFEB) is a master regulator of autophagy and lysosomal biogenesis. Therefore, activating TFEB and autophagy provides a novel strategy for AD treatment. We previously described that clomiphene citrate (CC) promotes nuclear translocation of TFEB and increases autophagy and lysosomal biogenesis. In this study, 7- and 3-month-old APP/PS1 mice were treated with TFEB agonist CC and assessed. The behavioral tests were performed using Morris water maze and open field test. Additional changes in amyloid-β pathology, autophagy, and inflammatory response were determined. We found that CC activated TFEB and the autophagy-lysosomal pathway in neuronal cells. Moreover, using mouse model of Alzheimer's disease, CC treatment promoted clearance of amyloid-β plaques and ameliorated cognitive function in both 7- and 3-month-old APP/PS1 mice. The CC-induced activation of TFEB occurs by promoting acetylation of TFEB for nuclear translocation. These findings provide a molecular mechanism for the TFEB-mediated activation of the autophagy-lysosome pathway by CC, which has the potential to be repurposed and applied in the treatment or prevention of AD.
Keywords: Alzheimer's disease; TFEB; autophagy; clomiphene citrate; lysosome.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Zhichao F., Lin-Xi W., Wei J., Bo L., Dongbo W. Targeting autophagy with small-molecule activators for potential therapeutic purposes. Eur. J. Med. Chem. 2023;260:115722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

