Distinct chemical structures inhibit the CEMIP hyaluronidase and promote oligodendrocyte progenitor cell maturation
- PMID: 39454959
- PMCID: PMC11742310
- DOI: 10.1016/j.jbc.2024.107916
Distinct chemical structures inhibit the CEMIP hyaluronidase and promote oligodendrocyte progenitor cell maturation
Abstract
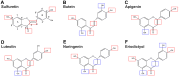
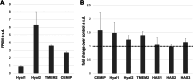
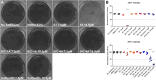
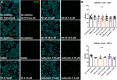
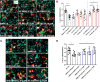
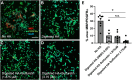
Growing evidence supports pathogenic roles for chronically elevated hyaluronidase activity in numerous conditions. Elevated expression of one such hyaluronidase, the Cell Migration Inducing and hyaluronan binding Protein (CEMIP), has been implicated in the pathogenesis and progression of several cancers as well as demyelinating diseases in the central nervous system (CNS). Developing effective and selective CEMIP inhibitors could therefore have efficacy in treating a variety of conditions where CEMIP is chronically elevated. Using two distinct screens for novel hyaluronidase inhibitors, we identified two synthetic thiocarbamates and one plant-derived flavonoid, sulfuretin, that effectively blocked CEMIP activity in live cells, including a tumorigenic cell line and primary cultures of oligodendrocyte progenitor cells (OPCs). None of these agents influenced cell proliferation, but they had differential dose-dependent and cell type-specific effects on cell survival. Furthermore, we found that each of these agents could promote oligodendrocyte maturation by OPCs in the presence of high molecular weight (>2 Mda) hyaluronan, the accumulation of which is linked to the inhibition of OPC maturation and remyelination failure in demyelinating diseases. These findings indicate that CEMIP can be inhibited through distinct chemical interactions and that CEMIP inhibitors have potential efficacy for treating demyelinating diseases or other conditions where CEMIP is elevated.
Keywords: CEMIP; flavonoids; hyaluronan; hyaluronidase; oligodendrocytes; sulfuretin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
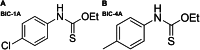
References
-
- De Luca C., Papa M. Matrix metalloproteinases, neural extracellular matrix, and central nervous system pathology. Prog. Mol. Biol. Transl. Sci. 2017;148:167–202. - PubMed
-
- Fawcett J.W. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain Res. 2015;218:213–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

