Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases
- PMID: 39455059
- PMCID: PMC11711082
- DOI: 10.1111/obr.13856
Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases
Abstract
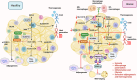
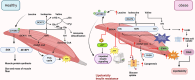
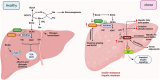
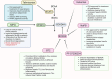
Branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential for maintaining physiological functions and metabolic homeostasis. However, chronic elevation of BCAAs causes metabolic diseases such as obesity, type 2 diabetes (T2D), and metabolic-associated fatty liver disease (MAFLD). Adipose tissue, skeletal muscle, and the liver are the three major metabolic tissues not only responsible for controlling glucose, lipid, and energy balance but also for maintaining BCAA homeostasis. Under obese and diabetic conditions, different pathogenic factors like pro-inflammatory cytokines, lipotoxicity, and reduction of adiponectin and peroxisome proliferator-activated receptors γ (PPARγ) disrupt BCAA metabolism, leading to excessive accumulation of BCAAs and their downstream metabolites in metabolic tissues and circulation. Mechanistically, BCAAs and/or their downstream metabolites, such as branched-chain ketoacids (BCKAs) and 3-hydroxyisobutyrate (3-HIB), impair insulin signaling, inhibit adipogenesis, induce inflammatory responses, and cause lipotoxicity in the metabolic tissues, resulting in multiple metabolic disorders. In this review, we summarize the latest studies on the metabolic regulation of BCAA homeostasis by the three major metabolic tissues-adipose tissue, skeletal muscle, and liver-and how dysregulated BCAA metabolism affects glucose, lipid, and energy balance in these active metabolic tissues. We also summarize therapeutic approaches to restore normal BCAA metabolism as a treatment for metabolic diseases.
Keywords: BCAAs; adipose tissue; diabetes and obesity; liver; metabolic tissues; skeletal muscle.
© 2024 The Author(s). Obesity Reviews published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

