Targeting CDCP1 boost CD8+ T cells-mediated cytotoxicity in cervical cancer via the JAK/STAT signaling pathway
- PMID: 39455095
- PMCID: PMC11529519
- DOI: 10.1136/jitc-2024-009416
Targeting CDCP1 boost CD8+ T cells-mediated cytotoxicity in cervical cancer via the JAK/STAT signaling pathway
Abstract
Background: Cervical cancer remains a global health challenge. The identification of new immunotherapeutic targets may provide a promising platform for advancing cervical cancer treatment.
Objective: This study aims to investigate the role of CUB domain-containing protein 1 (CDCP1) in cervical cancer progression and evaluate its potential as a therapeutic target.
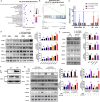
Methods: We performed comprehensive analyses using patient cohorts and preclinical models to examine the association between CDCP1 expression and cervical cancer prognosis. Then in immunodeficient and immunocompetent mouse models, we further investigated the impact of CDCP1 on the tumor immune microenvironment, focusing on its effects on tumor-infiltrating T cells, including cytotoxic T lymphocytes (CTLs) and regulatory T cells (Tregs). Mechanistic studies were performed to elucidate the pathways involved in CDCP1-mediated immune modulation, in particular its interaction with the T cell receptor CD6 and the activation of the JAK-STAT signaling pathway.
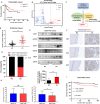
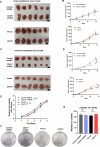
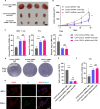
Results: Our results show that CDCP1 overexpression is associated with poor prognosis and T cell infliction in cervical cancer. Specifically, it affects the activity of CTLs and Tregs. Mechanistically, CDCP1 binds to CD6 and inhibits the JAK-STAT pathway of T cells. The study further demonstrates that targeting CDCP1 with the inhibitor 8-prenylnaringenin (8PN) effectively suppresses tumor growth in vivo and enhances antitumor immunity.
Conclusions: CDCP1 plays a critical role in cervical cancer progression by modulating the tumor immune microenvironment. Targeting CDCP1 offers a promising therapeutic strategy to improve the outcome of patients with cervical cancer.
Keywords: Cervical Cancer; Immunotherapy.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Delivery of Tapasin-modified CTL epitope peptide via cytoplasmic transduction peptide induces CTLs by JAK/STAT signaling pathway in vivo.Acta Biochim Biophys Sin (Shanghai). 2018 Feb 1;50(2):181-190. doi: 10.1093/abbs/gmx133. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 29340620
-
Inhibition of tumor metastasis: functional immune modulation of the CUB domain containing protein 1.Mol Pharm. 2010 Feb 1;7(1):245-53. doi: 10.1021/mp900236t. Mol Pharm. 2010. PMID: 19916495 Free PMC article.
-
Elevated CDCP1 predicts poor patient outcome and mediates ovarian clear cell carcinoma by promoting tumor spheroid formation, cell migration and chemoresistance.Oncogene. 2016 Jan 28;35(4):468-78. doi: 10.1038/onc.2015.101. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893298
-
JAK/STAT Signaling and Cervical Cancer: From the Cell Surface to the Nucleus.Genes (Basel). 2023 May 24;14(6):1141. doi: 10.3390/genes14061141. Genes (Basel). 2023. PMID: 37372319 Free PMC article. Review.
-
Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins.Cells. 2020 Oct 15;9(10):2297. doi: 10.3390/cells9102297. Cells. 2020. PMID: 33076315 Free PMC article. Review.
Cited by
-
Reverse-engineering the FLT3-PI3K/AKT axis to enhance TILs function and improve prognosis in ovarian and cervical cancers.J Ovarian Res. 2025 Jan 25;18(1):14. doi: 10.1186/s13048-025-01592-8. J Ovarian Res. 2025. PMID: 39863894 Free PMC article.
-
Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas.J Clin Med. 2025 Jul 19;14(14):5139. doi: 10.3390/jcm14145139. J Clin Med. 2025. PMID: 40725832 Free PMC article.
References
-
- Ferlay J, Ervik M, Lam F, et al. Lyon, France:: International Agency for Research on Cancer; 2024. Cancer observatory: cancer today; p. 8.https://gco.iarc.who.int/today Available.
-
- WHO Cervical cancer. 2023. https://www.who.int/zh/news-room/fact-sheets/detail/cervical-cancer Available.
-
- Vavassori A, Riva G, Spoto R, et al. High precision radiotherapy including intensity-modulated radiation therapy and pulsed-dose-rate brachytherapy for cervical cancer: a retrospective monoinstitutional study. J Contemp Brachytherapy. 2019;11:516–26. doi: 10.5114/jcb.2019.90478. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
