Polygenic risk, aspirin, and primary prevention of coronary artery disease
- PMID: 39455425
- PMCID: PMC11805692
- DOI: 10.1093/ehjcvp/pvae085
Polygenic risk, aspirin, and primary prevention of coronary artery disease
Abstract
Aims: Recent aspirin primary prevention trials failed to identify a net benefit of aspirin for preventing cardiovascular disease vs. the harms of bleeding. This study aimed to investigate whether a high-risk subgroup, individuals with elevated genetic predisposition to coronary artery disease (CAD), might derive more benefit than harm with aspirin, compared to those with lower genetic risk.
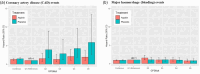
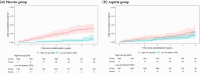
Methods and results: We performed genetic risk stratification of the Aspirin in Reducing Events in the Elderly (ASPREE) randomized controlled trial using a CAD polygenic risk score (GPSMult). For 12 031 genotyped participants (5974 aspirin, 6057 placebo) overall, we stratified them by GPSMult quintiles (q1-5), then examined risk of CAD (composite of myocardial infarction and coronary heart disease death) and bleeding events using Cox models. During a median 4.6 years of follow-up with randomization to 100 mg/day aspirin vs. placebo, 234 (1.9%) participants had CAD and 373 (3.1%) had bleeding events. In the overall cohort, aspirin resulted in higher bleeding risk [adjusted Hazard ratio (aHR) = 1.30 (1.06-1.61), P = 0.01] but no significant CAD reduction [aHR = 0.84 (0.64-1.09), P = 0.19]. However, among the highest quintile of polygenic risk (q5, top 20% of the GPSMult distribution), there was a 47% reduction in risk of CAD events with aspirin [aHR = 0.53 (0.31-0.90), P = 0.02] without increased bleeding risk [aHR = 1.05 (0.60-1.82), P = 0.88]. Interaction between the GPSMult and aspirin was significant for CAD (q5 vs. q1, P = 0.02) but not bleeding (P = 0.80).
Conclusion: The balance between net benefit and harm on aspirin in the primary prevention setting shifts favourably in individuals with an elevated genetic predisposition.
Keywords: Aspirin; Coronary artery disease; Genetic risk stratification; Polygenic risk score; Randomized controlled trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
A.M.T. has received research support from Bayer for materials in ASPREE, and honoraria for Advisory Board participation or lectures from Amgen, AstraZeneca, Boehringer-Ingelheim, and Pfizer. S.J.N. has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron and is a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, and Novo Nordisk. P.N. reports research grants from Allelica, Amgen, Apple, Boston Scientific, Genentech/Roche, and Novartis, personal fees from Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Creative Education Concepts, CRISPR Therapeutics, Eli Lilly & Co, Esperion Therapeutics, Foresite Capital, Foresite Labs, Genentech/Roche, GV, HeartFlow, Magnet Biomedicine, Merck, Novartis, Novo Nordisk, TenSixteen Bio, and Tourmaline Bio, equity in Bolt, Candela, Mercury, MyOme, Parameter Health, Preciseli, and TenSixteen Bio, and spousal employment at Vertex Pharmaceuticals, all unrelated to the present work. Bayer AG provided low-dose aspirin and placebo tablets for the clinical trial but had no other relationship with the work. S.Z. reports previous payments to institution (Monash University) from AstraZeneca, Boehringer Ingelheim, CSL Seqirus, Eli Lilly Australia, Moderna, MSD Australia, Sanofi and Novo Nordisk for participation in advisory and educational meetings. S.Z. is a Board Director of the Australian Clinical Trials Alliance and the Victorian Institute of Forensic Medicine, and has no declaration of interest specific to the research reported in this paper. H.S.B. is a consultant/advisor for Kaneka, Novartis, Arrowhead and Abbott, all unrelated to the present work. No other conflicts were reported.
Figures

Similar articles
-
Aspirin Dosing for Secondary Prevention of Atherosclerotic Cardiovascular Disease in Male and Female Patients: A Secondary Analysis of the ADAPTABLE Randomized Clinical Trial.JAMA Cardiol. 2024 Sep 1;9(9):808-816. doi: 10.1001/jamacardio.2024.1712. JAMA Cardiol. 2024. PMID: 38985488 Free PMC article. Clinical Trial.
-
Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular events.Cochrane Database Syst Rev. 2017 Dec 14;12(12):CD005158. doi: 10.1002/14651858.CD005158.pub4. Cochrane Database Syst Rev. 2017. PMID: 29240976 Free PMC article.
-
Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery.Cochrane Database Syst Rev. 2015 Feb 19;2015(2):CD000535. doi: 10.1002/14651858.CD000535.pub3. Cochrane Database Syst Rev. 2015. PMID: 25695213 Free PMC article.
-
Antithrombotic therapy to prevent cognitive decline in people with small vessel disease on neuroimaging but without dementia.Cochrane Database Syst Rev. 2022 Jul 14;7(7):CD012269. doi: 10.1002/14651858.CD012269.pub2. Cochrane Database Syst Rev. 2022. PMID: 35833913 Free PMC article.
-
Indobufen versus aspirin after percutaneous coronary intervention in elderly patients with acute coronary syndrome.BMC Cardiovasc Disord. 2025 Jul 7;25(1):495. doi: 10.1186/s12872-025-04843-0. BMC Cardiovasc Disord. 2025. PMID: 40619357 Free PMC article.
Cited by
-
Genomic risk prediction for depression in a large prospective study of older adults of European descent.Mol Psychiatry. 2025 Aug 6. doi: 10.1038/s41380-025-03145-3. Online ahead of print. Mol Psychiatry. 2025. PMID: 40770434
References
-
- Hennekens CH, Dyken ML, Fuster V. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the. Am Heart Assoc Circ 1997;96:2751–2753. - PubMed
-
- Ridker PM. Should aspirin be used for primary prevention in the post-statin era?. N Engl J Med 2018;379:1572–1574. - PubMed
-
- Mcneil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–1518. - PMC - PubMed
-
- ASCEND Study Collaborative Group . Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–1539. - PubMed
-
- Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet North Am Ed 2018;392:1036–1046. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL166962/HL/NHLBI NIH HHS/United States
- U19 AG062682/AG/NIA NIH HHS/United States
- U01 AG029824/AG/NIA NIH HHS/United States
- U01 HG011719/HG/NHGRI NIH HHS/United States
- R01 HL142711/HL/NHLBI NIH HHS/United States
- U01AG029824/AG/NIA NIH HHS/United States
- Flagship Cluster
- K08 HL168238/HL/NHLBI NIH HHS/United States
- 334047/National Health and Medical Research Council of Australia
- 5U01AG29824-02/NH/NIH HHS/United States
- 102604/National Heart Foundation Future Leader Fellowship
- R01HL142711/HL/NHLBI NIH HHS/United States

