Impact of Continuous Infusion Meropenem PK/PD Target Attainment on C-Reactive Protein Dynamics in Critically Ill Patients With Documented Gram-Negative Hospital-Acquired or Ventilator-Associated Pneumonia
- PMID: 39455501
- PMCID: PMC11573875
- DOI: 10.1007/s40262-024-01436-6
Impact of Continuous Infusion Meropenem PK/PD Target Attainment on C-Reactive Protein Dynamics in Critically Ill Patients With Documented Gram-Negative Hospital-Acquired or Ventilator-Associated Pneumonia
Abstract
Background and objective: Population pharmacokinetic/pharmacodynamic (PK/PD) modelling of antibiotics including C-reactive protein (C-RP) dynamics could be helpful in predicting the efficacy of antimicrobials. We developed a PK/PD model for assessing the impact of continuous infusion (CI) meropenem PK/PD target attainment on C-RP dynamics in critically ill patients with documented Gram-negative hospital- (HAP) or ventilator-acquired pneumonia (VAP).
Methods: Patients were grouped according to the type of antibiotic treatment received [meropenem monotherapy; meropenem plus empirical anti-MRSA (methicillin-resistant Staphylococcus aureus) therapy; meropenem in combination with another anti-Gram-negative active agent; meropenem plus a targeted anti-MRSA therapy]. A one-compartment population PK model of CI meropenem was developed by including all patients. A full C-RP production inhibition model was developed for fitting the PD data by including only patients receiving meropenem monotherapy or meropenem plus empirical anti-MRSA therapy. Monte Carlo simulations explored the relationship between the type of PK/PD target attainment of CI meropenem, defined as optimal (steady-state plasma concentration [Css] to minimum inhibitory concentration [MIC] ratio = 4-8), quasi-optimal (Css/MIC = 1-4) and sub-optimal (Css/MIC < 1) and the magnitude of C-RP production inhibition over time.
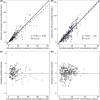
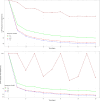
Results: A total of 64 patients providing 211 meropenem concentrations were included in the PK analysis, whereas 47 patients providing 328 C-RP data were included in the PD model. Simulations showed that optimal PK/PD target attainment was associated with the highest and most rapid C-RP production inhibition (44% and 56% at days 2 and 4, respectively). Conversely, sub-optimal PK/PD target attainment was shown to be almost ineffective (< 5% at day 4 and < 10% at day 10).
Conclusion: Our PK/PD model predicted that attaining optimal PK/PD target with CI meropenem may grant prompt and intense C-RP decrease among critically ill patients receiving targeted monotherapy for Gram-negative HAP/VAP, thus anticipating efficacy.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

