Asia-Pacific Real-World Evolocumab Use, LDL-C Reduction, Physician Goals, and Patient Perceptions: HALES Observational Study
- PMID: 39455535
- PMCID: PMC11607257
- DOI: 10.1007/s40119-024-00384-3
Asia-Pacific Real-World Evolocumab Use, LDL-C Reduction, Physician Goals, and Patient Perceptions: HALES Observational Study
Abstract
Introduction: Real-world data are needed to understand the effectiveness of new therapeutic options for low-density lipoprotein cholesterol (LDL-C) reduction in Asia-Pacific clinical practice. Description of evolocumab use among adults with establisHed Atherosclerotic cardiovascuLar diseasE or hypercholesterolemia in ASia-Pacific region (HALES) was performed to better understand characteristics of and clinical decision-making for adults with established atherosclerotic cardiovascular disease/hypercholesterolemia after local evolocumab approval.
Methods: The HALES observational study, conducted at 33 sites (Hong Kong, Thailand, South Korea, Singapore, Taiwan, and Australia) comprised (1) chart review of patients who received evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i), and (2) physician/patient survey and one-time data collection of patients with high cardiovascular risk initiating evolocumab or initiating/continuing non-PCSK9i lipid-lowering therapy. Patients could only enroll in (1) or (2).
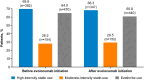
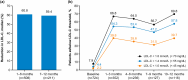
Results: Chart review included 724 very high-risk patients initiating evolocumab from regulatory approval to 2021. From median baseline LDL-C of 3.2 mmol/L (123.7 mg/dL), patients had a median percent change in LDL-C of - 60.8% at 1-6 months. Goal achievement increased from 7.9% to 69.8% for < 1.8 mmol/L (< 70 mg/dL) and 4.4% to 57.8% for < 1.4 mmol/L (< 55 mg/dL) from baseline to 12 months. In the one-time data collection, more patients had ≥ 1.8 mmol/L (≥ 70 mg/dL) baseline LDL-C in the evolocumab vs non-PCSK9i group (95.2% and 48.5%, respectively). Surveys found that physicians applied guideline-recommended treatment targets, and patients demonstrated gaps in understanding cardiovascular risk.
Conclusion: Real-world, Asia-Pacific data showed that LDL-C reduction after initiating evolocumab was consistent with that observed in other clinical trials and patient populations. Graphical abstract available for this article.·.
Keywords: Asia; Australia; Cardiovascular event reduction; Chart review; Evolocumab; LDL-C reduction; Observational research; Patient-reported outcome; Physician feedback; Real-world evidence.
Plain language summary
Excessive levels of low-density lipoprotein cholesterol (LDL-C) in the blood can lead to heart disease. Evolocumab, a drug that can lower LDL-C levels, was approved for use in the Asia–Pacific region. This real-world study was conducted to understand how effective evolocumab was in lowering LDL-C in patients in the Asia–Pacific region. Doctor and patient perspectives regarding disease management and risk awareness, respectively, were obtained. The study was conducted in two parts in six Asia–Pacific countries/regions. In the first part, investigators examined the medical charts of 724 patients who had started taking evolocumab plus their usual lipid-lowering therapies. The investigators assessed the change in LDL-C levels from the time patients started taking evolocumab. In the second part, doctors were surveyed on how they managed lipid levels in patients with a high risk of developing heart disease. Patients with an increased risk of developing heart disease were surveyed to understand their awareness of heart disease risk. Results from part 1 revealed that LDL-C levels fell by approximately 60% within 6 months of starting evolocumab therapy. Results from part 2 revealed that most doctors followed recommended guidelines for lipid measurement and management. The patient survey showed that approximately one in two patients were unaware of their total cholesterol level or what their optimal LDL-C levels should be. This real-world study found that evolocumab lowered LDL-C levels in Asia–Pacific patients similarly to clinical trials. Patients need more education to understand and better manage their risk for heart disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Hung-Fat Tse reports research funding from Abbott, AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Medtronic, Novartis, Pfizer, and Sanofi. Hung-Yu Chang, Kian Keong Poh, Karam Kostner, and Pisit Hutayanon report that they have nothing to declare. David Colquhoun reports research funding from Amgen, Pfizer, AstraZeneca, Novartis, Sanofi, Bayer, and Novo Nordisk. Jung-Sun Kim reports receiving proctoring fees from Abbott Vascular. Meejin Cho, Jeff Lange, Kamlanathan Kodiappan, and Saikiran Leekha are employees and stockholders of Amgen Inc. Ethics Approval: Informed consent was required for patient’s participation in the study. Study protocol and informed consent forms were reviewed and approved by the institutional review board/independent ethics committee (see Supplementary Materials, Supplemental Table S2 for full list by site). Central IRBs were used for sites in Australia and Singapore; local IRBD/ethics committees were used in all other sites. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Figures
References
-
- World Heart Federation. What is cardiovascular disease? https://world-heart-federation.org/what-is-cvd/. Accessed Oct 15, 2023.
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. - PMC - PubMed
-
- Bruckert E, Catapano AL, Farnier M, et al. The clinician’s handbook: dyslipidaemia and atherosclerosis prevention, diagnosis and treatment 2019 - update 2023. https://eas-society.org/publications/clinicians-handbook/. Accessed Apr 1, 2024.
LinkOut - more resources
Full Text Sources

