Antibodies targeting the Crimean-Congo Hemorrhagic Fever Virus nucleoprotein protect via TRIM21
- PMID: 39455551
- PMCID: PMC11511847
- DOI: 10.1038/s41467-024-53362-7
Antibodies targeting the Crimean-Congo Hemorrhagic Fever Virus nucleoprotein protect via TRIM21
Abstract
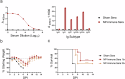
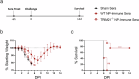
Crimean-Congo Hemorrhagic Fever Virus (CCHFV) is a negative-sense RNA virus spread by Hyalomma genus ticks across Europe, Asia, and Africa. CCHF disease begins as a non-specific febrile illness which may progress into a severe hemorrhagic disease with no widely approved or highly efficacious interventions currently available. Recently, we reported a self-replicating, alphavirus-based RNA vaccine that expresses the CCHFV nucleoprotein and is protective against lethal CCHFV disease in mice. This vaccine induces high titers of non-neutralizing anti-NP antibodies and we show here that protection does not require Fc-gamma receptors or complement. Instead, vaccinated mice deficient in the intracellular Fc-receptor TRIM21 were unable to control the infection despite mounting robust CCHFV-specific immunity. We also show that passive transfer of NP-immune sera confers significant TRIM21-dependent protection against lethal CCHFV challenge. Together our data identifies TRIM21-mediated mechanisms as the Fc effector function of protective antibodies against the CCHFV NP and provides mechanistic insight into how vaccines against the CCHFV NP confer protection.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
J.E. has equity interest in HDT Bio and is co-inventor on U.S. patent application no. 62/993,307 “Compositions and methods for delivery of RNA” pertaining to formulations for RNA delivery. DWH, JE and HF are inventors on U.S. patent application number 63/365,015 “Replicating RNA vaccine for Crimean-Congo hemorrhagic fever virus” regarding the repRNA for use against CCHFV. The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

