One-carbon-mediated purine synthesis underlies temozolomide resistance in glioblastoma
- PMID: 39455562
- PMCID: PMC11511812
- DOI: 10.1038/s41419-024-07170-y
One-carbon-mediated purine synthesis underlies temozolomide resistance in glioblastoma
Abstract
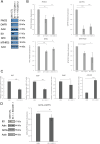
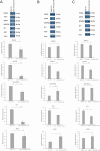
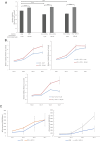
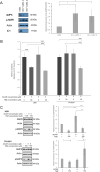
Glioblastoma accounts for nearly half of all primary malignant brain tumors in adults, and despite an aggressive standard of care, including excisional surgery and adjuvant chemoradiation, recurrence remains universal, with an overall median survival of 14.6 months. Recent work has revealed the importance of passenger mutations as critical mediators of metabolic adaptation in cancer progression. In our previous work, we identified a role for the epigenetic modifier ID-1 in temozolomide resistance in glioblastoma. Here, we show that ID-1-mediated glioblastoma tumourigenesis is accompanied by upregulation of one-carbon (1-C) mediated de novo purine synthesis. ID-1 knockout results in a significant reduction in the expression of 1-C metabolism and purine synthesis enzymes. Analysis of glioblastoma surgical specimens at initial presentation and recurrence reveals that 1-C purine synthesis metabolic enzymes are enriched in recurrent glioblastoma and that their expression correlates with a shorter time to tumor recurrence. Further, we show that the 1-C metabolic phenotype underlies proliferative capacity and temozolomide resistance in glioblastoma cells. Supplementation with exogenous purines restores proliferation in ID-1-deficient cells, while inhibition of purine synthesis with AICAR sensitizes temozolomide-resistant glioblastoma cells to temozolomide chemotherapy. Our data suggest that the metabolic phenotype observed in treatment-resistant glioma cells is a potential therapeutic target in glioblastoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

