Asymmetric Büchner reaction and arene cyclopropanation via copper-catalyzed controllable cyclization of diynes
- PMID: 39455569
- PMCID: PMC11511906
- DOI: 10.1038/s41467-024-53605-7
Asymmetric Büchner reaction and arene cyclopropanation via copper-catalyzed controllable cyclization of diynes
Abstract
The asymmetric Büchner reaction and related arene cyclopropanations represent one type of the powerful methods for enantioselective dearomatization. However, examples of asymmetric Büchner reactions via a non-diazo approach are quite scarce, and the related arene cyclopropanation based on alkynes has not been reported. Herein, we disclose an asymmetric Büchner reaction and the related arene cyclopropanation by copper-catalyzed controllable cyclization of N-propargyl ynamides via vinyl cation intermediates, leading to chiral tricycle-fused cycloheptatrienes and benzonorcaradienes in high yields and enantioselectivities. Importantly, this protocol represents an asymmetric arene cyclopropanation reaction of alkynes and an asymmetric Büchner reaction based on vinyl cations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


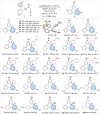
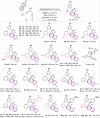


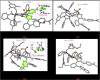
References
-
- Liu, Y.-Z., Song, H., Zheng, C. & You, S.-L. Cascade asymmetric dearomative cyclization reactions via transition-metal catalysis. Nat. Synth.1, 203–216 (2022). - DOI
-
- Sheng, F.-T., Wang, J.-Y., Tan, W., Zhang, Y.-C. & Shi, F. Progresses in organocatalytic asymmetric dearomatization reactions of indole derivatives. Org. Chem. Front.7, 3967–3998 (2020). - DOI
LinkOut - more resources
Full Text Sources

