The regulatory T cell-selective interleukin-2 receptor agonist rezpegaldesleukin in the treatment of inflammatory skin diseases: two randomized, double-blind, placebo-controlled phase 1b trials
- PMID: 39455575
- PMCID: PMC11511931
- DOI: 10.1038/s41467-024-53384-1
The regulatory T cell-selective interleukin-2 receptor agonist rezpegaldesleukin in the treatment of inflammatory skin diseases: two randomized, double-blind, placebo-controlled phase 1b trials
Abstract
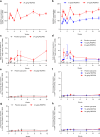
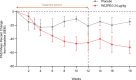
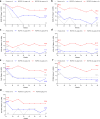
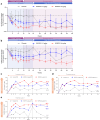
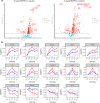
Regulatory T cell (Treg) impairment is implicated in the pathogenesis of chronic inflammatory diseases, but relatively little is known about the therapeutic potential of Treg restoration. Here we present clinical evidence for the Treg-selective interleukin-2 receptor agonist rezpegaldesleukin (REZPEG) in two randomized, double-blind, placebo-controlled Phase 1b trials in patients with moderate-to-severe atopic dermatitis (AD) (NCT04081350) or chronic plaque psoriasis (PsO) (NCT04119557). Key inclusion criteria for AD included an Eczema Area and Severity Index (EASI) score ≥ 16 and a validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) ≥ 3, and for PsO included a Psoriasis Area and Severity Index (PASI) score of ≥ 12 and a static Physician's Global Assessment (sPGA) score of ≥ 3. REZPEG is safe and well-tolerated and demonstrates consistent pharmacokinetics in participants receiving subcutaneous doses of 10 to 12 µg/kg or 24 µg/kg once every 2 weeks for 12 weeks, meeting the primary and secondary objectives, respectively. AD patients receiving the higher dose demonstrate an 83% improvement in EASI score after 12 weeks of treatment. EASI improvement of ≥ 75% (EASI-75) and vIGA-AD responses are maintained for 36 weeks after treatment discontinuation in 71% and 80% of week 12 responders, respectively. These exploratory clinical improvements are accompanied by sustained increases in CD25bright Tregs. REZPEG thus represents a homeostatic approach to cutaneous disease therapy and holds clinical potential in providing long-term, treatment-free disease control.
© 2024. The Author(s).
Conflict of interest statement
JS has received honoraria as a consultant and/or advisory board member for Abbvie, Aldena, Amgen, AObiome, Apollo, Arcutis, Arena, Asana, Aslan, Attovia, BioMX, Biosion, Bodewell, Boehringer-Ingelheim, Bristol Meyers Squibb, Cara, Castle Biosciences, Celgene, Connect Biopharma, Corevitas, Dermavant, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Invea, Kiniksa, Leo Pharma, My-Or Diagnostics, Nektar, Novartis, Optum, Pfizer, RAPT, Recludix, Regeneron, Sanofi-Genzyme, Shaperon, TARGET-RWE, Union, UpToDate; speaker for Abbvie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, Sanofi-Genzyme; stock/stock options for Connect, Verdant; institution received grants from Galderma, Incyte, Pfizer DR has consulted, spoken for, or conducted trials for the following companies: AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer-Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Nektar, Novartis, Pfizer, RAPT, Regeneron, Recludix, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, VielaBio, Zura Bio. RC has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Amgen, AnaptysBio, Apogee Therapeutics, Arcutis, Argenx, ASLAN Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Eli Lilly and Company, FIDE, Formation Bio, Galderma, Genentech, GSK, Incyte, LEO Pharma, L’Oréal, Nektar Therapeutics, Novartis, Opsidio, Pfizer Inc., RAPT, Regeneron, Sanofi, Sitryx, Takeda, TRexBio, and UCB. TB has been a speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, Amagma, AnaptysBio, AOBiom, Apogee, Arena, Aristea, Artax, Asana Biosciences, ASLAN pharma, Astria, Attovia, Bayer Health, Biofilm control, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Daichi-Sanyko, Dermavant, DICE Therapeutics, Domain Therapeutics, DS Pharma, EQRx, Galderma, Galapagos, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L´Oréal, MSD, Medac, Micreos, Nektar, Novartis, Numab, OM-Pharma, Overtone, Pfizer, Pierre Fabre, Q32bio, RAPT, Samsung Bioepis, Sanofi/Regeneron, TIRmed, UCB, Union Therapeutics, UPStream Bio, YUHAN. He is founder and chairman of the board of the non-profit biotech Davos Biosciences within the international Kühne-Foundation. SS has received research funding from Eli Lilly and Company, Amgen, Galderma, HighLight Therapeutics, Encube, Cara Therapeutics, Vyne, Novan, AstraZeneca. Honoria for Nektar. Consulting/Advisory Role for Nektar. LB has been a consultant and/or investigator for Abbvie, Allakos, Amgen, Arcutis, Arena Pharmaceuticals, AstraZeneca, Astria Therapeutics, Evelo Biosciences, Escient Pharma, Galderma, Incyte, Invea Therapeutics, Janssen, LEO Pharma, Merck, Nektar Therapeutics, Novartis, Numab Therapeutics, Pfizer, Rapt Therapeutics, Regeneron Pharmaceuticals Inc., Ribon Therapeutics, Sanofi-Aventis/Genzyme, Sitryx Therapeutics, Stealth BioTherapeutics, Trevi Therapeutics, UCB Pharma, Union therapeutics, and Xencor. MG has been an investigator, speaker and/or advisor for: AbbVie, Acelyrin, Amgen, Akros, Arcutis, Aristea, AnaptysBio, Apogee, Bausch Health, BMS, Boehringer Ingelheim, Cara, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, InMagene, JAMP Pharma, Janssen, Kyowa Kirin, LEO Pharma, L’Oreal, MedImmune, Meiji, Moonlake, Nektar, Nimbus, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Tarsus, Takeda, UCB, Union, Ventyx and Vyne. SC, CF, DY, YL, BK, TM, MT, and JZ are employees and stockholders at Nektar Therapeutics. MT also has a leadership role and is a shareholder in Enzo Biochem and RayzeBio. CS and AN are shareholders at Eli Lilly and Company. AN is a shareholder at Recludix Pharma. JL is a stockholder in Eli Lilly and Company.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

