ETFDH mutation involves excessive apoptosis and neurite outgrowth defect via Bcl2 pathway
- PMID: 39455656
- PMCID: PMC11511830
- DOI: 10.1038/s41598-024-75286-4
ETFDH mutation involves excessive apoptosis and neurite outgrowth defect via Bcl2 pathway
Abstract
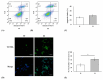
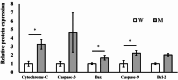
The most common mutation in southern Chinese individuals with late-onset multiple acyl-coenzyme A dehydrogenase deficiency (MADD; a fatty acid metabolism disorder) is c.250G > A (p.Ala84Thr) in the electron transfer flavoprotein dehydrogenase gene (ETFDH). Various phenotypes, including episodic weakness or rhabdomyolysis, exercise intolerance, and peripheral neuropathy, have been reported in both muscular and neuronal contexts. Our cellular models of MADD exhibit neurite growth defects and excessive apoptosis. Given that axonal degeneration and neuronal apoptosis may be regulated by B-cell lymphoma (BCL)-2 family proteins and mitochondrial outer membrane permeabilization through the activation of proapoptotic molecules, we measured the expression levels of proapoptotic BCL-2 family proteins (e.g., BCL-2-associated X protein and p53-upregulated modulator of apoptosis), cytochrome c, caspase-3, and caspase-9 in NSC-34 cells carrying the most common ETFDH mutation. The levels of these proteins were higher in the mutant cells than in the wide-type cells. Subsequent treatment of the mutant cells with coenzyme Q10 downregulated activated protein expression and mitigated neurite growth defects. These results suggest that the activation of the BCL-2/mitochondrial outer membrane permeabilization/apoptosis pathway promotes apoptosis in cellular models of MADD and that coenzyme Q10 can reverse this effect. Our findings aid the development of novel therapeutic strategies for reducing axonal degeneration and neuronal apoptosis in MADD.
Keywords: Apoptosis; Bcl-2; Carnitine; Coenzyme Q10; ETFDH; MOMP; Multiple acyl-coenzyme A dehydrogenase deficiency; Neuropathy; Riboflavin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Indo, Y., Glassberg, R., Yokota, I. & Tanaka, K. Molecular characterization of variant alpha-subunit of electron transfer flavoprotein in three patients with glutaric acidemia type II–and identification of glycine substitution for valine-157 in the sequence of the precursor, producing an unstable mature protein in a patient. Am. J. Hum. Genet.49, 575–580 (1991). - PMC - PubMed
-
- Freneaux, E., Sheffield, V. C., Molin, L., Shires, A. & Rhead, W. J. Glutaric acidemia type II. Heterogeneity in beta-oxidation flux, polypeptide synthesis, and complementary DNA mutations in the alpha subunit of electron transfer flavoprotein in eight patients. J. Clin. Invest.90, 1679–1686 (1992). - PMC - PubMed
-
- Beard, S. E., Goodman, S. I., Bemelen, K. & Freman, F. E. Characterization of a mutation that abolishes quinone reduction by electron transfer flavoprotein-ubiquinone oxidoreductase. Hum. Mol. Genet.4, 157–161 (1995). - PubMed
-
- Goodman, S. I., Binard, R. J., Woontner, M. R. & Freman, F. E. Glutaric acidemia type II: gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol. Genet. Metab.77, 86–90 (2002). - PubMed
-
- Ghisla, S. & Thorpe, C. Acyl-CoA dehydrogenases. A mechanistic overview. Eur. J. Biochem.271, 494–508 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

