An innovative electrical neurostimulation approach to mimic reflexive urination control in spinal cord injury models
- PMID: 39455718
- PMCID: PMC11511940
- DOI: 10.1038/s41598-024-76499-3
An innovative electrical neurostimulation approach to mimic reflexive urination control in spinal cord injury models
Abstract
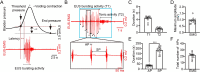
Neurogenic lower urinary tract dysfunction (NLUTD) is a frequent consequence of spinal cord injury (SCI), leading to symptoms that significantly impact quality of life. Although many life-saving techniques are available, current treatment strategies for managing NLUTD still exhibit limitations and drawbacks. Here, we introduce a new electrical neuromodulation strategy involving electrical stimulation of the major pelvic ganglion (MPG) to initiate bladder contraction, in conjunction with innovative programmable (IPG) electrical stimulation on the pudendal nerve (PN) to induce external urethral sphincter (EUS) relaxation in freely moving or anesthetized SCI mice. Furthermore, we conducted the void spot assay, and cystometry coupled with EUS electromyography (EMG) recordings to evaluate voiding function, and monitor bladder pressure and EUS activity. Our findings demonstrate that our novel electrical neuromodulation approach effectively triggers coordinated bladder muscle contraction and EUS relaxation, effectively counteracting SCI-induced NLUTD. Additionally, this electrical neuromodulation method enhances voiding efficiency, closely resembling natural reflexive urination in SCI mice. Thus, our study offers a promising electrical neurostimulation approach aimed at restoring physiological coordination and potentially offering personalized treatment for improving voiding efficiency in individuals with SCI-associated NLUTD.
Keywords: Electrical neurostimulation; Major pelvic ganglion; Neurogenic lower urinary tract dysfunction; Pudendal nerve; Spinal cord injury; Urination control.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R752-8. doi: 10.1152/ajpregu.00450.2015. Epub 2016 Jan 27. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26818058 Free PMC article.
-
Effects of pudendal neuromodulation on bladder function in chronic spinal cord-injured rats.J Formos Med Assoc. 2016 Sep;115(9):703-13. doi: 10.1016/j.jfma.2015.07.010. Epub 2015 Sep 19. J Formos Med Assoc. 2016. PMID: 26386674
-
Restoring both continence and micturition after chronic spinal cord injury by pudendal neuromodulation.Exp Neurol. 2021 Jun;340:113658. doi: 10.1016/j.expneurol.2021.113658. Epub 2021 Feb 24. Exp Neurol. 2021. PMID: 33639209
-
Electrical nerve stimulation to promote micturition in spinal cord injury patients: A review of current attempts.Neurourol Urodyn. 2016 Mar;35(3):365-70. doi: 10.1002/nau.22730. Epub 2015 Feb 8. Neurourol Urodyn. 2016. PMID: 25663151 Review.
-
Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury.J Spinal Cord Med. 2015 Mar;38(2):135-46. doi: 10.1179/2045772314Y.0000000299. Epub 2015 Jan 13. J Spinal Cord Med. 2015. PMID: 25582564 Free PMC article. Review.
Cited by
-
Neurogenic Voiding Dysfunction in Spinal Cord Injury and Stroke: Urodynamic Evaluation, Functional Classification, and Therapeutic Strategies.Cureus. 2025 Aug 4;17(8):e89348. doi: 10.7759/cureus.89348. eCollection 2025 Aug. Cureus. 2025. PMID: 40909072 Free PMC article. Review.
-
SST neurons in the periaqueductal gray regulate urination and bladder function.Commun Biol. 2025 Apr 21;8(1):639. doi: 10.1038/s42003-025-08069-w. Commun Biol. 2025. PMID: 40259086 Free PMC article.
References
-
- Benarroch, E. E. Neural control of the bladder: Recent advances and neurologic implications. Neurology 75, 1839–1846 (2010). - PubMed
-
- Li, H., Nahm, N., Borchert, A., Wong, P. & Atiemo, H. Contemporary treatment of detrusor sphincter dyssynergia: A systematic review. Curr. Bladder Dysfunct. Rep. 13, 206–214 (2018).
-
- Mangera, A. et al. An updated systematic review and statistical comparison of standardised mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur. Urol. 65, 981–990 (2014). - PubMed
-
- Terpenning, M. S., Allada, R. & Kauffman, C. A. Intermittent urethral catheterization in the elderly. J. Am. Geriatr. Soc. 37, 411–416 (1989). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

