Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy
- PMID: 39455854
- PMCID: PMC11588664
- DOI: 10.1038/s41375-024-02444-y
Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy
Abstract
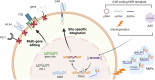
Chimeric Antigen Receptor (CAR)-T-cell therapy has revolutionized cancer immune therapy. However, challenges remain including increasing efficacy, reducing adverse events and increasing accessibility. Use of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology can effectively perform various functions such as precise integration, multi-gene editing, and genome-wide functional regulation. Additionally, CRISPR screening using large-scale guide RNA (gRNA) genetic perturbation provides an unbiased approach to understanding mechanisms underlying anti-cancer efficacy of CAR T-cells. Several emerging CRISPR tools with high specificity, controllability and efficiency are useful to modify CAR T-cells and identify new targets. In this review we summarize potential uses of the CRISPR system to improve results of CAR T-cells therapy including optimizing efficacy and safety and, developing universal CAR T-cells. We discuss challenges facing CRISPR gene editing and propose solutions highlighting future research directions in CAR T-cell therapy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: RPG is a consultant to Antengene Biotech LLC, Ascentage Pharma Group and NexImmune Inc.; Medical Director, FFF Enterprises Inc.; A speaker for Janssen Pharma and Hengrui Pharma; Board of Directors: Russian Foundation for Cancer Research Support; and Scientific Advisory Boards, Nanexa AB and StemRad Ltd. AH receives research support from Novartis, BMS, Incyte and Pfizer.
Figures





References
-
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. - PubMed
-
- US Food and Drug Administration: KYMRIAH (tisagenlecleucel). 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ.... Accessed 9 Oct 2024.
-
- US Food and Drug Administration: YESCARTA (axicabtagene ciloleucel). 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ.... Accessed 9 Oct 2024.
-
- US Food and Drug Administration: TECARTUS (brexucabtagene autoleucel). 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ.... Accessed 9 Oct 2024.
-
- US Food and Drug Administration: BREYANZI (lisocabtagene maraleucel). 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ.... Accessed 9 Oct 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

