Chemogenetics with PSAM4-GlyR decreases excitability and epileptiform activity in epileptic hippocampus
- PMID: 39455855
- PMCID: PMC11946892
- DOI: 10.1038/s41434-024-00493-7
Chemogenetics with PSAM4-GlyR decreases excitability and epileptiform activity in epileptic hippocampus
Abstract
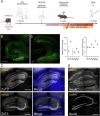
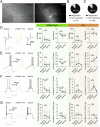
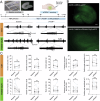
Despite the availability of new drugs on the clinics in recent years, drug-resistant epilepsy remains an unresolved challenge for healthcare, and one-third of epilepsy patients remain refractory to anti-seizure medications. Gene therapy in experimental models has emerged as effective treatment targeting specific neuronal populations in the epileptogenic focus. When combined with an external chemical activator using chemogenetics, it also becomes an "on-demand" treatment. Here, we evaluate a targeted and specific chemogenetic therapy, the PSAM/PSEM system, which holds promise as a potential candidate for clinical application in treating drug-resistant epilepsy. We show that the inert ligand uPSEM817, which selectively activates the chloride-permeable channel PSAM4-GlyR, effectively reduces the number of depolarization-induced action potentials in vitro. This effect is likely due to the shunting of depolarizing currents, as evidenced by decreased membrane resistance in these cells. In organotypic slices, uPSEM817 decreased the number of bursts and peak amplitude of events of spontaneous epileptiform activity. Although administration of uPSEM817 in vivo did not significantly alter electrographic seizures in a male mouse model of temporal lobe epilepsy, it did demonstrate a strong trend toward reducing the frequency of interictal epileptiform discharges. These findings indicate that PSAM4-GlyR-based chemogenetics holds potential as an anti-seizure strategy, although further refinement is necessary to enhance its efficacy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: As described in the material and methods section, the experimental procedures performed were approved by the Malmö/Lund Animal Research Ethics Board, ethical permit number 2998/2020-m, and conducted in agreement with the Swedish Animal Welfare Agency regulations and the EU Directive 2010/63/EU for animal experiments. Organotypic slices experiments were conducted according to European Union Guidelines (2012/707/EU) and to the Portuguese legislative action (DL 113/2013) for the protection of animals used for scientific purposes. The methods described were approved by “iMM’s Institutional Animal Welfare Body (ORBEA-iMM, Lisboa, Portugal) and authorized by the Portuguese authority for Animal Welfare (Direção Geral de Alimentação e Veterinária - DGAV).
Figures


References
-
- Toda Y, Kobayashi K, Hayashi Y, Inoue T, Oka M, Ohtsukaet Y. Effects of intravenous diazepam on high-frequency oscillations in EEGs with CSWS. Brain Dev. 2013;35:540–7. - PubMed
-
- Peng B-W, Justice JA, Zhang K, Li J-X, He X-H, Sanchez RM. Gabapentin promotes inhibition by enhancing hyperpolarization-activated cation currents and spontaneous firing in hippocampal CA1 interneurons. Neurosci Lett. 2011;494:19–23. - PubMed
-
- Braga MF, Aroniadou-Anderjaska V, Li H, Rogawski MA. Topiramate reduces excitability in the basolateral amygdala by selectively inhibiting GluK1 (GluR5) kainate receptors on interneurons and positively modulating GABAA receptors on principal neurons. J Pharmacol Exp Ther. 2009;330:558–66. - PubMed
-
- Ylinen A, Valjakka A, Lahtinen H, Miettinen R, Freund TF, Riekkinen P. Vigabatrin pre-treatment prevents hilar somatostatin cell loss and the development of interictal spiking activity following sustained simulation of the perforant path. Neuropeptides. 1991;19:205–11. - PubMed
MeSH terms
Grants and funding
- Primus/26/MED/011/Charles University | Lékařská Fakulta v Plzni, Univerzita Karlova (Faculty of Medicine in Pilsen, Charles University)
- H2020-WIDESPREAD-2020-5, Agreement ID 952455/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- H2020-MSCA-ITN-2016, Agreement ID 722779/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 2016-02605/Vetenskapsrådet (Swedish Research Council)
- 2017-00921/Vetenskapsrådet (Swedish Research Council)
LinkOut - more resources
Full Text Sources
Medical

