Inflammation impacts androgen receptor signaling in basal prostate stem cells through interleukin 1 receptor antagonist
- PMID: 39455902
- PMCID: PMC11511867
- DOI: 10.1038/s42003-024-07071-y
Inflammation impacts androgen receptor signaling in basal prostate stem cells through interleukin 1 receptor antagonist
Abstract
Chronic prostate inflammation in patients with benign prostate hyperplasia (BPH) correlates with the severity of symptoms. How inflammation contributes to prostate enlargement and/or BPH symptoms and the underlying mechanisms remain unclear. In this study, we utilize a unique transgenic mouse model that mimics chronic non-bacterial prostatitis in men and investigate the impact of inflammation on androgen receptor (AR) in basal prostate stem cells (bPSC) and their differentiation in vivo. We find that inflammation significantly enhances AR levels and activity in bPSC. More importantly, we identify interleukin 1 receptor antagonist (IL-1RA) as a crucial regulator of AR in bPSC during inflammation. IL-1RA is one of the top molecules upregulated by inflammation, and inhibiting IL-1RA reverses the enhanced AR activity in organoids derived from inflamed bPSC. Additionally, IL-1RA appears to activate AR by counteracting IL-1α's inhibitory effect. Furthermore, using a lineage tracing model, we observe that inflammation induces bPSC proliferation and differentiation into luminal cells even under castrate conditions, indicating that AR activation driven by inflammation is sufficient to promote bPSC proliferation and differentiation. Taken together, our study uncovers mechanisms through which inflammation modulates AR signaling in bPSC and induces bPSC luminal differentiation that may contribute to prostate hyperplasia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


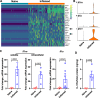

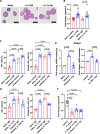
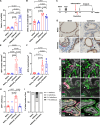

Update of
-
Inflammation Impacts Androgen Receptor Signaling in Basal Prostate Stem Cells Through Interleukin 1 Receptor Antagonist.Res Sq [Preprint]. 2023 Dec 15:rs.3.rs-3539806. doi: 10.21203/rs.3.rs-3539806/v1. Res Sq. 2023. Update in: Commun Biol. 2024 Oct 25;7(1):1390. doi: 10.1038/s42003-024-07071-y. PMID: 38168414 Free PMC article. Updated. Preprint.
References
-
- Gandaglia, G. et al. The role of prostatic inflammation in the development and progression of benign and malignant diseases. Curr. Opin. Urol.27, 99–106 (2017). - PubMed
-
- De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol.13, 613–626 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- UM1 TR004402/TR/NCATS NIH HHS/United States
- R01DK126478/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 DK084454/DK/NIDDK NIH HHS/United States
- R01 DK126478/DK/NIDDK NIH HHS/United States
- R01DK084454/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

