Effectiveness and safety of prehospital tranexamic acid in patients with trauma: an updated systematic review and meta-analysis with trial sequential analysis
- PMID: 39455930
- PMCID: PMC11515107
- DOI: 10.1186/s12873-024-01119-2
Effectiveness and safety of prehospital tranexamic acid in patients with trauma: an updated systematic review and meta-analysis with trial sequential analysis
Abstract
Background: The use of prehospital tranexamic acid (TXA) in patients with trauma has attracted considerable attention. This systematic review and meta-analysis aimed to provide the best evidence for clinicians.
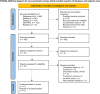
Methods: All related literature in PubMed, Embase, and the Cochrane Central Register of Controlled Trials (Central) databases were searched systematically from their establishment to July 1, 2023. The outcome measures included 24-hour and 28-30-day mortality and adverse events (multiple organ dysfunction syndrome, acute respiratory distress syndrome, thrombotic events, and infection events). The Revised Cochrane Risk of Bias Tool for Randomized Trials was used to evaluate the quality of the randomized controlled trials (RCTs). The Methodological Index for Nonrandomized Studies (MINORS) was used to evaluate the risk of bias in non-RCTs. The required information size was estimated using trial sequential analysis. The Grading of Recommendations, Assessment, Development, and Evaluation approach was used to evaluate the evidence quality.
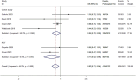
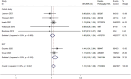
Results: Eleven studies (comprising 11,259 patients) were included; two of these were RCTs. The overall risks of bias were low in the RCTs. ROBINS-I risk of bias was Moderate in 3 studies, serious in 5 studies, and critical in 1 study. A significant reduction in 24-hour mortality was observed (odds ratio [OR], 0.82; 95% confidence interval [CI], 0.71-0.94). A subgroup analysis that included only RCTs revealed that prehospital TXA was associated with reduced 28-30-day mortality (OR, 0.80; 95% CI, 0.66-0.97) and increased risks of thromboembolism (OR, 1.22; 95% CI, 1.03-1.44) and infection (OR, 1.13; 95% CI, 1.00-1.28) events. The blood products for transfusion decreased by 2.3 units on average (weighted mean difference [WMD], - 2.30; 95%CI, - 3.59 to - 1.01).
Conclusions: This updated systematic review showed that prehospital TXA reduced the 24-hour and 28-38-day mortality and blood transfusion but increased the risks of infection and thromboembolism in patients with trauma. Future RCTs with larger and more homogeneous samples will help verify our results.
Keywords: Meta-analysis; Prehospital; Tranexamic acid; Trauma; Trial sequential analysis.
© 2024. The Author(s).
Conflict of interest statement
The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Figures





References
-
- Perel P, Prieto-Merino D, Shakur H, Clayton T, Lecky F, Bouamra O, Russell R, Faulkner M, Steyerberg EW, Roberts I. Predicting early death in patients with traumatic bleeding: development and validation of prognostic model. BMJ (Clinical Res ed). 2012;345:e5166. 10.1136/bmj.e5166. - DOI - PMC - PubMed
-
- Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. The Journal of trauma 2008; 64(6): 1459-63; discussion 1463-5. 10.1097/TA.0b013e318174e8bc - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

