Skeletal myotubes expressing ALS mutant SOD1 induce pathogenic changes, impair mitochondrial axonal transport, and trigger motoneuron death
- PMID: 39455931
- PMCID: PMC11505737
- DOI: 10.1186/s10020-024-00942-4
Skeletal myotubes expressing ALS mutant SOD1 induce pathogenic changes, impair mitochondrial axonal transport, and trigger motoneuron death
Abstract
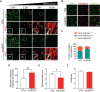
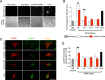
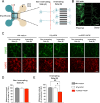
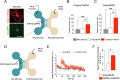
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the loss of motoneurons (MNs), and despite progress, there is no effective treatment. A large body of evidence shows that astrocytes expressing ALS-linked mutant proteins cause non-cell autonomous toxicity of MNs. Although MNs innervate muscle fibers and ALS is characterized by the early disruption of the neuromuscular junction (NMJ) and axon degeneration, there are controversies about whether muscle contributes to non-cell-autonomous toxicity to MNs. In this study, we generated primary skeletal myotubes from myoblasts derived from ALS mice expressing human mutant SOD1G93A (termed hereafter mutSOD1). Characterization revealed that mutSOD1 skeletal myotubes display intrinsic phenotypic and functional differences compared to control myotubes generated from non-transgenic (NTg) littermates. Next, we analyzed whether ALS myotubes exert non-cell-autonomous toxicity to MNs. We report that conditioned media from mutSOD1 myotubes (mutSOD1-MCM), but not from control myotubes (NTg-MCM), induced robust death of primary MNs in mixed spinal cord cultures and compartmentalized microfluidic chambers. Our study further revealed that applying mutSOD1-MCM to the MN axonal side in microfluidic devices rapidly reduces mitochondrial axonal transport while increasing Ca2 + transients and reactive oxygen species (i.e., H2O2). These results indicate that soluble factor(s) released by mutSOD1 myotubes cause MN axonopathy that leads to lethal pathogenic changes.
Keywords: ALS; Axonopathy; Mitochondria; Motoneuron; Muscle; Myotubes; Pathology.
© 2024. The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures

Update of
-
Skeletal myotubes expressing ALS mutant SOD1 induce pathogenic changes, impair mitochondrial axonal transport, and trigger motoneuron death.bioRxiv [Preprint]. 2024 May 26:2024.05.24.595817. doi: 10.1101/2024.05.24.595817. bioRxiv. 2024. Update in: Mol Med. 2024 Oct 25;30(1):185. doi: 10.1186/s10020-024-00942-4. PMID: 38826246 Free PMC article. Updated. Preprint.
Similar articles
-
Skeletal myotubes expressing ALS mutant SOD1 induce pathogenic changes, impair mitochondrial axonal transport, and trigger motoneuron death.bioRxiv [Preprint]. 2024 May 26:2024.05.24.595817. doi: 10.1101/2024.05.24.595817. bioRxiv. 2024. Update in: Mol Med. 2024 Oct 25;30(1):185. doi: 10.1186/s10020-024-00942-4. PMID: 38826246 Free PMC article. Updated. Preprint.
-
AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice.Skelet Muscle. 2024 Jul 24;14(1):17. doi: 10.1186/s13395-024-00349-z. Skelet Muscle. 2024. PMID: 39044305 Free PMC article.
-
Novel Dual Mechanism GRT-X Agonist Acting on Kv7 Potassium Channel/Translocator Protein Receptor Prevents Motoneuron Degeneration Following Exposure to Mouse and Human Amyotrophic Lateral Sclerosis/Frontotemporal Dementia Astrocyte-Conditioned Media.ACS Chem Neurosci. 2025 Aug 6;16(15):2887-2900. doi: 10.1021/acschemneuro.5c00197. Epub 2025 Jul 17. ACS Chem Neurosci. 2025. PMID: 40671688 Free PMC article.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
-
Motoneuronal and muscle-selective removal of ALS-related misfolded proteins.Biochem Soc Trans. 2013 Dec;41(6):1598-604. doi: 10.1042/BST20130118. Biochem Soc Trans. 2013. PMID: 24256261 Review.
Cited by
-
Exploring the Role of Axons in ALS from Multiple Perspectives.Cells. 2024 Dec 17;13(24):2076. doi: 10.3390/cells13242076. Cells. 2024. PMID: 39768167 Free PMC article. Review.
-
Amyotrophic Lateral Sclerosis: Focus on Cytoplasmic Trafficking and Proteostasis.Mol Neurobiol. 2025 Aug;62(8):10091-10117. doi: 10.1007/s12035-025-04831-7. Epub 2025 Apr 3. Mol Neurobiol. 2025. PMID: 40180687 Free PMC article. Review.
References
-
- Aguirre T, van Den Bosch L, Goetschalckx K, et al. Increased sensitivity of fibroblasts from amyotrophic lateral sclerosis patients to oxidative stress. Ann Neurol. 1998;43:452–7. 10.1002/ana.410430407. - PubMed
-
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–28. 10.1038/nrneurol.2013.203. - PubMed
-
- Aurnhammer C, Haase M, Muether N, et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Part b Methods. 2012;23:18–28. 10.1089/hgtb.2011.034. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

