Synergistic effects of bacteriophage cocktail and antibiotics combinations against extensively drug-resistant Acinetobacter baumannii
- PMID: 39455951
- PMCID: PMC11515142
- DOI: 10.1186/s12879-024-10081-0
Synergistic effects of bacteriophage cocktail and antibiotics combinations against extensively drug-resistant Acinetobacter baumannii
Abstract
Background: The extensively drug-resistant (XDR) strains of Acinetobacter baumannii have become a major cause of nosocomial infections, increasing morbidity and mortality worldwide. Many different treatments, including phage therapy, are attractive ways to overcome the challenges of antibiotic resistance.
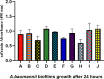
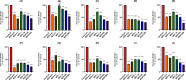
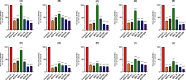
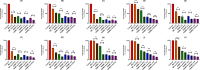
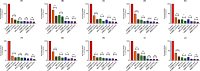
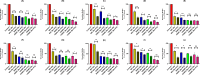
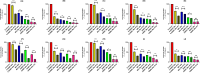
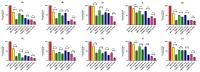
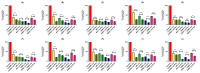
Methods: This study investigates the biofilm formation ability of 30 XDR A. baumannii isolates and the efficacy of a cocktail of four tempetate bacteriophages (SA1, Eve, Ftm, and Gln) and different antibiotics (ampicillin/sulbactam, meropenem, and colistin) in inhibiting and degrading the biofilms of these strains.
Results: The majority (83.3%) of the strains exhibited strong biofilm formation. The bacteriophage cocktail showed varying degrees of effectiveness against A. baumannii biofilms, with higher concentrations generally leading to more significant inhibition and degradation rates. The antibiotics-bacteriophage cocktail combinations also enhanced the inhibition and degradation of biofilms.
Conclusion: The findings suggested that the bacteriophage cocktail is an effective tool in combating A. baumannii biofilms, with its efficacy depending on the concentration. Combining antibiotics with the bacteriophage cocktail improved the inhibition and removal of biofilms, indicating a promising strategy for managing A. baumannii infections. These results contribute to our understanding of biofilm dynamics and the potential of bacteriophage cocktails as a novel therapeutic approach to combat antibiotic-resistant bacteria.
Keywords: Acinetobacter baumannii; Bacteriophage; Extensively drug-resistant; Siphovirus; Synergistic effect.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mohebi S, Golestani-Hotkani Z, Foulad-Pour M, Nazeri P, Mohseni F, Hashemizadeh Z, et al. Characterization of integrons, extended spectrum beta lactamases and genetic diversity among uropathogenic Escherichia coli isolates from Kerman, South East of Iran. Iran J Microbiol. 2023;15:616. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

