The effect of prebiotic fibre on the gut microbiome and surgical outcomes in patients with prosthetic joint infection (PENGUIN) - study protocol for a randomised, double-blind, placebo-controlled trial (ACTRN12623001273673)
- PMID: 39455990
- PMCID: PMC11515416
- DOI: 10.1186/s12937-024-01034-z
The effect of prebiotic fibre on the gut microbiome and surgical outcomes in patients with prosthetic joint infection (PENGUIN) - study protocol for a randomised, double-blind, placebo-controlled trial (ACTRN12623001273673)
Abstract
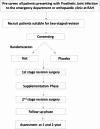
Background: Prosthetic Joint Infection (PJI) is the most devastating complication of arthroplasty surgery and affects 1-5% of patients. Despite strict adherence to aseptic protocols and preventive measures, infection is the most common reason for revision arthroplasty, and the incidence is increasing. Treatment of PJI is challenging and often requires repeated major surgeries with sequentially poor results. The continued occurrence of PJI, and persistence after treatment, brings into question the current treatment paradigm. Preclinical evidence suggests a link between altered gut health and the risk of PJI in arthroplasty patients. Resistant starches helps to restore gut physiology by enhancing the beneficial microbiome and producing short-chain fatty acids, which have several health-conferring properties. The primary aim of this study is to investigate the effect of a commercially available prebiotic fibre formulation on the gut microbiome in PJI patients planned for a two-stage revision surgery.
Methods: A double-blind placebo-controlled trial will assess the effect of 8-week supplementation of a commercially available prebiotic supplement in patients presenting with first-time PJI undergoing two-stage revision surgery. The supplementation phase will start after the first stage revision, and 80 patients will be randomised to receive either a test product (34 g of resistant starch) or a placebo (custard powder) daily for eight weeks. Stool and blood specimens will be collected at baseline, four weeks and eight weeks after the first-stage surgery and once at second-stage surgery. Gut microbiome profile, inflammatory cytokines and gut permeability biomarkers will be measured. Tissue specimens will be collected intra-operatively during first and second-stage surgeries. Baseline dietary patterns and gut symptoms will be recorded using validated questionnaires. Treatment outcomes will be reported for both cohorts using the Delphi criterion at one and two years after second-stage surgery.
Discussion: This will be the first study to investigate the relationship between gut health optimisation and preventing PJI recurrence in arthroplasty patients. If supplementation with resistant starch improves gut health and reduces systemic inflammation, optimising the gut microbiome will be a recommended preoperative management strategy for arthroplasty patients.
Trial registration no: ACTRN12623001273673.
Keywords: Arthroplasty; Gut dysbiosis; Prebiotic fibre; Prosthetic joint infection; Resistant starch; Two-stage revision; Zonulin levels.
© 2024. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Registry AOANJR. Hip, knee & shoulder arthroplasty: 2023 Annual report. Adelaide: AOA 2023.
-
- Health AIo W. Cancer in Australia 2019. Cancer series no. 119. Cat. No. CAN 123. AIHW Canberra; 2019.
-
- Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95:2177–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical