Targeting FAP-positive chondrocytes in osteoarthritis: a novel lipid nanoparticle siRNA approach to mitigate cartilage degeneration
- PMID: 39456041
- PMCID: PMC11515236
- DOI: 10.1186/s12951-024-02946-y
Targeting FAP-positive chondrocytes in osteoarthritis: a novel lipid nanoparticle siRNA approach to mitigate cartilage degeneration
Abstract
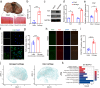
Background: Osteoarthritis (OA) is a common joint disease that leads to chronic pain and functional limitations. Recent research has revealed soluble fibroblast activation protein (FAP) secreted from OA synovium could degrade type II collagen (Col2) in cartilage to promote the progression of OA. This study aimed to reveal the role of FAP from chondrocytes in OA and develop a novel lipid nanoparticle (LNP)-FAP siRNA delivery system for OA treatment.
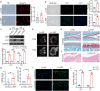
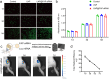
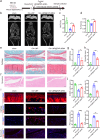
Methods: The expression of FAP in the cartilage of knee OA patients was investigated using [68 Ga]Ga-FAPI-04 PET in vivo and immunofluorescence, western blotting, and RT-qPCR in vitro. Cell senescence was determined by senescence-associated β-galactosidase (SA-β-Gal) assay after FAP overexpressing or knockdown in chondrocytes. An OA model with chondrocyte-specific FAP knockout mice was applied to investigate the role of FAP in chondrocyte senescence and OA development. The therapeutic effects of lipid nanoparticle (LNP) @FAP siRNA on cartilage degeneration were evaluated in the rat OA model.
Results: Our study found that higher [68 Ga]Ga-FAPI-04 uptake was detected in knee OA patients by PET/CT scan. FAP mRNA and protein levels were highly expressed in OA-damaged cartilage. Moreover, we found that overexpression of FAP promotes chondrocyte senescence, and the genetic knockout of FAP in chondrocytes alleviates OA. Knockdown FAP by siRNA could alleviate chondrocyte senescence and suppress the NF-κB pathway to reduce the senescence-associated secretory phenotype (SASP). In the rat model of OA, intraarticular injection of LNP@FAP siRNA can reduce senescent cells and ameliorate cartilage destruction.
Conclusion: FAP-positive chondrocytes play a significant role in the pathogenesis of OA. Targeting these cells selectively has the potential to mitigate the progression of the disease. Our study provides valuable insights into the intraarticular injection of LNP@FAP siRNA as a promising strategy for the treatment of OA.
Keywords: FAP; LNP; Osteoarthritis; Senescence; [68 Ga]Ga-FAPI-04 PET/CT.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

