Whole milk protein powder separated by low-temperature nanofiltration membrane administration alleviates sepsis-induced myopathy
- PMID: 39456082
- PMCID: PMC11515193
- DOI: 10.1186/s12986-024-00862-4
Whole milk protein powder separated by low-temperature nanofiltration membrane administration alleviates sepsis-induced myopathy
Abstract
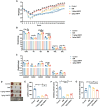
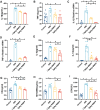
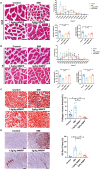
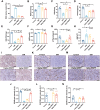
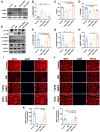
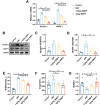
Sepsis-induced myopathy (SIM) has been recognized as a critical risk factor for the development of acquired muscle weakness among patients in the intensive care unit. These individuals frequently encounter inadequate dietary intake and malnutrition. With the aggravation of the severity of the person's condition, leading to increased skeletal muscle protein breakdown and reduced synthesis, which is an urgent problem to be solved in clinical nutritional treatment. Whole milk protein powder (WMPP) has promising bioactive nutrients and holds promising potential for enhancing skeletal muscle mass. The study was designed to delve into the potential effects and mechanisms of WMPP intervention for increaseing skeletal muscle mass on SIM mice. Our results clearly show that the intervention with WMPP can significantly improve the exercise capacity and skeletal muscle mass in SIM mice. It significantly increases the diameter and cross-sectional area (CSA) of skeletal muscle fibers, while effectively reducing the excessive aggregation of collagen fibers and the abnormal accumulation of adipose tissue in the skeletal muscle of SIM mice. Moreover, WMPP intervention also significantly alleviated the oxidative stress status of mitochondria, which subsequently enhanced the expression of mitochondrial metabolic enzymes. The mechanism may be associated with decreased AMPK phosphorylation in skeletal muscle tissue and simultaneously increased phosphorylation of mTOR, p70S6K1, and 4EBP-1 in SIM mice. In summary, the WMPP intervention significantly enhances exercise capacity and skeletal muscle mass while mitigating the oxidative stress status of mitochondria. Furthermore, it regulates skeletal muscle anabolism via the AMPK/mTOR signaling pathway in SIM mice.
Keywords: AMPK/mTOR signal pathway; Mitochondria function; Sepsis-induced myopathy; Whole milk protein powder.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation.J Appl Physiol (1985). 2018 Sep 1;125(3):715-722. doi: 10.1152/japplphysiol.00984.2016. Epub 2018 Jun 28. J Appl Physiol (1985). 2018. PMID: 29952246
-
AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity.FASEB J. 2014 Jul;28(7):3211-24. doi: 10.1096/fj.14-250449. Epub 2014 Mar 20. FASEB J. 2014. PMID: 24652947
-
Reduced metabolic capacity in fast and slow skeletal muscle via oxidative stress and the energy-sensing of AMPK/SIRT1 in malnutrition.Physiol Rep. 2021 Mar;9(5):e14763. doi: 10.14814/phy2.14763. Physiol Rep. 2021. PMID: 33650806 Free PMC article.
-
Protein intake and amino acid supplementation regulate exercise recovery and performance through the modulation of mTOR, AMPK, FGF21, and immunity.Nutr Res. 2019 Dec;72:1-17. doi: 10.1016/j.nutres.2019.06.006. Epub 2019 Jul 2. Nutr Res. 2019. PMID: 31672317 Review.
-
Sepsis-induced myopathy.Crit Care Med. 2009 Oct;37(10 Suppl):S354-67. doi: 10.1097/CCM.0b013e3181b6e439. Crit Care Med. 2009. PMID: 20046121 Free PMC article. Review.
Cited by
-
The cardioprotective effect of whey protein against thioacetamide-induced toxicity through its antioxidant, anti-inflammatory, and anti-apoptotic effects in male albino rats.Front Vet Sci. 2025 May 19;12:1590722. doi: 10.3389/fvets.2025.1590722. eCollection 2025. Front Vet Sci. 2025. PMID: 40458758 Free PMC article.
References
-
- Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology. 2006;67:1421–5. 10.1212/01.wnl.0000239826.63523.8e. - PubMed
-
- Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M, Matot B, Carlier PG, Latronico N, Huchet C, Lafoux A, Sharshar T, Ricchetti M, Chrétien F. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. 2015;6:10145. 10.1038/ncomms10145. - PMC - PubMed
-
- Boczkowski J, Lisdero CL, Lanone S, Carreras MC, Aubier M, Poderoso JJ. Peroxynitrite-mediated mitochondrial dysfunction. Biol Signals Recept. 2001;10:66–80. 10.1159/000046876. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

