The wonders of X-PDT: an advance route to cancer theranostics
- PMID: 39456085
- PMCID: PMC11520131
- DOI: 10.1186/s12951-024-02931-5
The wonders of X-PDT: an advance route to cancer theranostics
Abstract
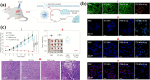
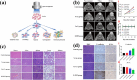
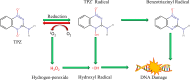
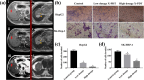
Global mortality data indicates cancer as the second-leading cause of death worldwide. Therefore, there's a pressing need to innovate effective treatments to address this significant medical and societal challenge. In recent years, X-ray-induced photodynamic therapy (X-PDT) has emerged as a promising advancement, revolutionizing traditional photodynamic therapy (PDT) for deeply entrenched malignancies by harnessing penetrating X-rays as external stimuli. Recent developments in X-ray photodynamic therapy have shown a trend toward minimizing radiation doses to remarkably low levels after the proof-of-concept demonstration. Early detection and real-time monitoring are crucial aspects of effective cancer treatment. Sophisticated X-ray imaging techniques have been enhanced by the introduction of X-ray luminescence nano-agents, alongside contrast nanomaterials based on X-ray attenuation. X-ray luminescence-based in vivo imaging offers excellent detection sensitivity and superior image quality in deep tissues at a reasonable cost, due to unhindered penetration and unimpeded auto-fluorescence of X-rays. This review emphasizes the significance of X-ray responsive theranostics, exploring their mechanism of action, feasibility, biocompatibility, and promising prospects in imaging-guided therapy for deep-seated tumors. Additionally, it discusses promising applications of X-PDT in treating breast cancer, liver cancer, lung cancer, skin cancer, and colorectal cancer.
Keywords: Deep tumors; ROS; Theranostic; X-PDT; X-ray responsive imaging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

