Repurposing Nitazoxanide for Potential Treatment of Rare Disease Lymphangioleiomyomatosis
- PMID: 39456169
- PMCID: PMC11506457
- DOI: 10.3390/biom14101236
Repurposing Nitazoxanide for Potential Treatment of Rare Disease Lymphangioleiomyomatosis
Abstract
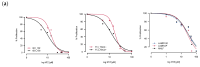
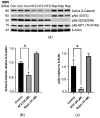
Lymphangioleiomyomatosis (LAM) is a rare genetic lung disease. Unfortunately, treatment with the mTORC1 inhibitor Rapamycin only slows disease progression, and incomplete responses are common. Thus, there remains an urgent need to identify new targets for the development of curative LAM treatments. Nitazoxanide (NTZ) is an orally bioavailable antiprotozoal small molecule drug approved for the treatment of diarrhea caused by Giardia lamblia or Cryptosporidium parvum in children and adults, with a demonstrated mTORC1 inhibitory effect in several human cell lines. NTZ's excellent safety profile characterized by its more than 20 years of clinical use makes it a promising candidate for repurposing. Our rationale for this study was to further investigate NTZ's effect using in vitro and in vivo LAM models and to elucidate the underlying molecular mechanism beyond mTORC1 inhibition. For this purpose, we investigated cell proliferation, cell viability, and changes in protein phosphorylation and expression in primary human cell cultures derived from LAM lung samples before translating our results into a syngeneic mouse model utilizing Tsc2-null cells. NTZ reduced cell growth for all tested cell lines at a dose of about 30 µM. Lower doses than that had no effect on cell viability, but doses above 45 µM lowered the viability by about 10 to 15% compared to control. Interestingly, our western blot revealed no inhibition of mTORC1 and only a mild effect on active ß-Catenin. Instead, NTZ had a pronounced effect on reducing pAkt. In the mouse model, prophylactic NTZ treatment via the intraperitoneal and oral routes had some effects on reducing lung lesions and improving body weight retention, but the results remain inconclusive.
Keywords: Nitazoxanide; drug repurposing; lung disease; lymphangioleiomyomatosis; rare disease.
Conflict of interest statement
Authors Stella Bähr, Ryan W. Rue, Carly J. Smith and Dirk Pleimes were employed by the company Biosputnik LLC. H.K. holds a patent for Nitazoxanide for use in lymphangioleiomyomatosis (EP3709985A1; US11547699B2).
Figures




References
-
- Rout P., Zamora E.A.M., Aeddula N.R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 27 May 2024)]. Tuberous Sclerosis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538492/ - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

