Design of a Robust Flow Cytometric Approach for Phenotypical and Functional Analysis of Human Monocyte Subsets in Health and Disease
- PMID: 39456184
- PMCID: PMC11506830
- DOI: 10.3390/biom14101251
Design of a Robust Flow Cytometric Approach for Phenotypical and Functional Analysis of Human Monocyte Subsets in Health and Disease
Abstract
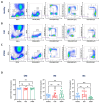
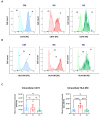
Human monocytes can be subdivided into phenotypically and functionally different classical, intermediate and non-classical monocytes according to the cell surface expression of CD14 and CD16. A precise identification and characterisation of monocyte subsets is necessary to unravel their role in inflammatory diseases. Here, we compared three different flow cytometric strategies (A-C) and found that strategy C, which included staining against CD11b, HLA-DR, CD14 and CD16, followed by several gating steps, most reliably identified monocyte subtypes in blood samples from healthy volunteers and from patients with stable coronary heart disease (CHD) or ST-elevation myocardial infarction (STEMI). Additionally, we established a fixation and permeabilisation protocol to enable the analysis of intracellular markers. We investigated the phagocytosis of lipid nanoparticles, the uptake of 2-NBD-glucose and the intracellular levels of CD74 and HLA-DM. This revealed that classical and intermediate monocytes from patients with STEMI showed the highest uptake of 2-NBD-glucose, whereas classical and intermediate monocytes from patients with CHD took up the largest amounts of lipid nanoparticles. Interestingly, intermediate monocytes had the highest expression level of HLA-DM. Taken together, we present a robust flow cytometric approach for the identification and functional characterisation of monocyte subtypes in healthy humans and patients with diseases.
Keywords: MHC class II pathway; coronary heart disease; flow cytometry; gating; glucose uptake; monocyte subsets; myocardial infarction; phagocytosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

