Postoperative Electroacupuncture Boosts Cognitive Function Recovery after Laparotomy in Mice
- PMID: 39456207
- PMCID: PMC11506768
- DOI: 10.3390/biom14101274
Postoperative Electroacupuncture Boosts Cognitive Function Recovery after Laparotomy in Mice
Abstract
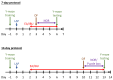
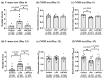
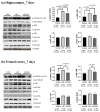
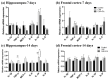
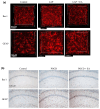
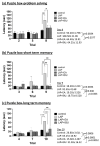
Postoperative cognitive dysfunction (POCD) is a common complication that affects memory, executive function, and processing speed postoperatively. The pathogenesis of POCD is linked to excessive neuroinflammation and pre-existing Alzheimer's disease (AD) pathology. Previous studies have shown that acupuncture improves cognition in the early phase of POCD. However, POCD can last for longer periods (up to weeks and years). The long-term effects of acupuncture are unknown. In this study, we hypothesized that electroacupuncture (EA) could reduce inflammation and cognitive dysfunction induced by laparotomy over a longer period. We characterized the effects of postoperative EA on cognitive changes and investigated the underlying molecular mechanisms in mice. Laparotomy was performed in 3-month-old mice followed by daily EA treatment for 2 weeks. Our data indicated that laparotomy induced prolonged impairment in memory and executive functions, which were mitigated by postoperative EA. EA also reduced tau phosphorylation and suppressed the activation of tau-related kinases and glia, with effects comparable to ibuprofen. These findings demonstrate the beneficial effects of EA in a mouse model of POCD, suggesting that EA's ability to suppress neuroinflammation may contribute to its protective effects. In conclusion, EA may be a viable non-pharmacological intervention for managing POCD in different phases of the medical condition.
Keywords: Chinese medicine; acupuncture; cognition; inflammation; memory; surgery; tau.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

