Structural and Dynamical Properties of Nucleic Acid Hairpins Implicated in Trinucleotide Repeat Expansion Diseases
- PMID: 39456210
- PMCID: PMC11505666
- DOI: 10.3390/biom14101278
Structural and Dynamical Properties of Nucleic Acid Hairpins Implicated in Trinucleotide Repeat Expansion Diseases
Abstract
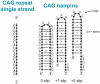
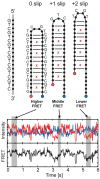
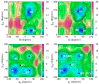
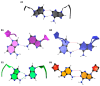
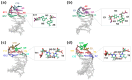
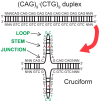
Dynamic mutations in some human genes containing trinucleotide repeats are associated with severe neurodegenerative and neuromuscular disorders-known as Trinucleotide (or Triplet) Repeat Expansion Diseases (TREDs)-which arise when the repeat number of triplets expands beyond a critical threshold. While the mechanisms causing the DNA triplet expansion are complex and remain largely unknown, it is now recognized that the expandable repeats lead to the formation of nucleotide configurations with atypical structural characteristics that play a crucial role in TREDs. These nonstandard nucleic acid forms include single-stranded hairpins, Z-DNA, triplex structures, G-quartets and slipped-stranded duplexes. Of these, hairpin structures are the most prolific and are associated with the largest number of TREDs and have therefore been the focus of recent single-molecule FRET experiments and molecular dynamics investigations. Here, we review the structural and dynamical properties of nucleic acid hairpins that have emerged from these studies and the implications for repeat expansion mechanisms. The focus will be on CAG, GAC, CTG and GTC hairpins and their stems, their atomistic structures, their stability, and the important role played by structural interrupts.
Keywords: CAG and GAC; CTG and GTC; expansion diseases; hairpin structure; molecular dynamics simulations; single-molecule FRET; smFRET; trinucleotide repeats.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wells R.D., Ashizawa T. Genetic Instabilities and Neurological Diseases. Elsevier; Boston, MA, USA: 2006.
-
- Fleischer B. Uber mytotoische Dystrophie mit Katarakt. Albrecht Von Graefes Arch. Klin. Exp. Optalmol. 1918;96:905–914.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

